

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50442114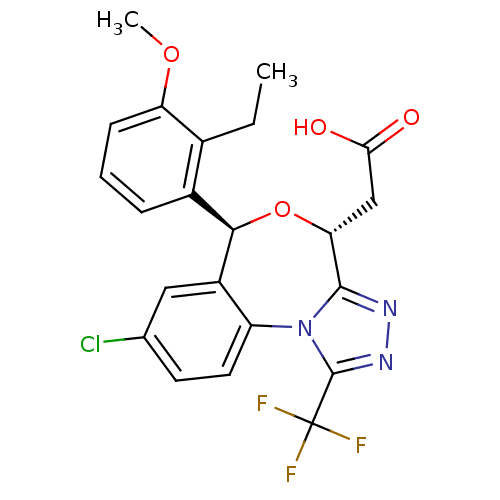 (CHEMBL2441090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442115 (CHEMBL2440128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442113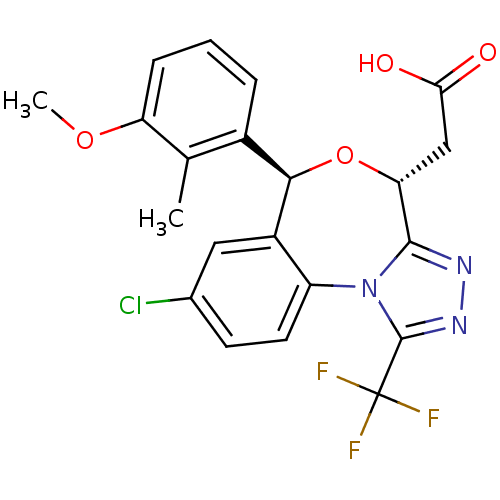 (CHEMBL2441083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442112 (CHEMBL2441084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442116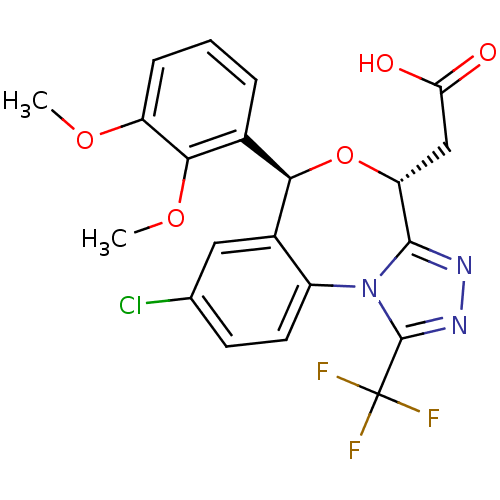 (CHEMBL2441089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442117 (CHEMBL2441088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442118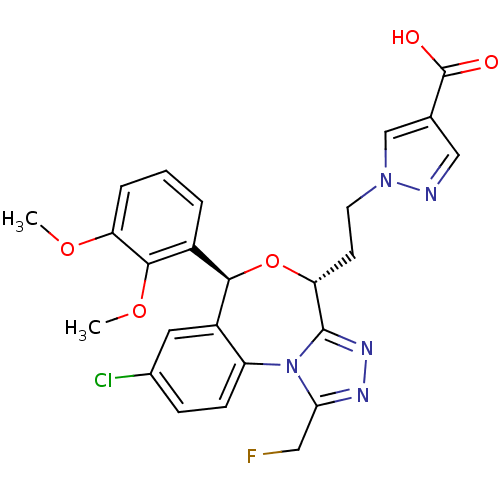 (CHEMBL2441087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442119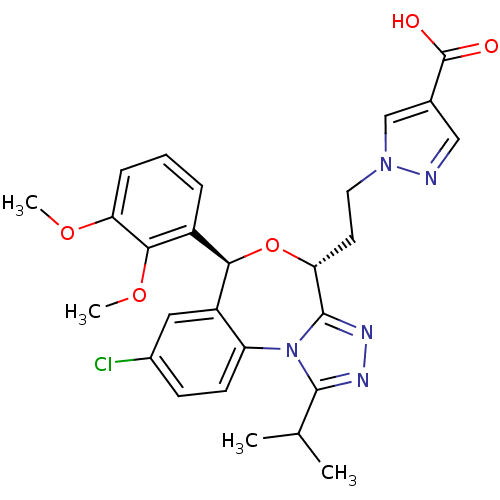 (CHEMBL2441086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442120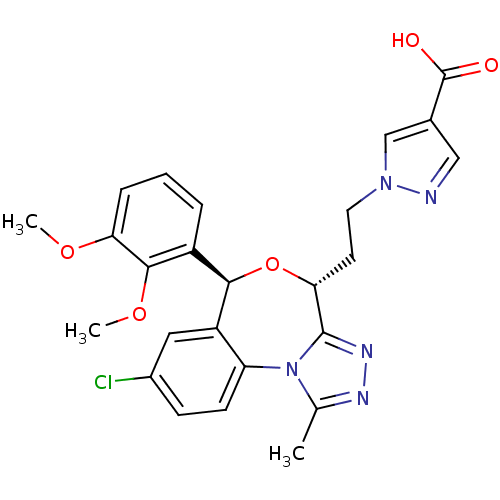 (CHEMBL2441085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||