

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470139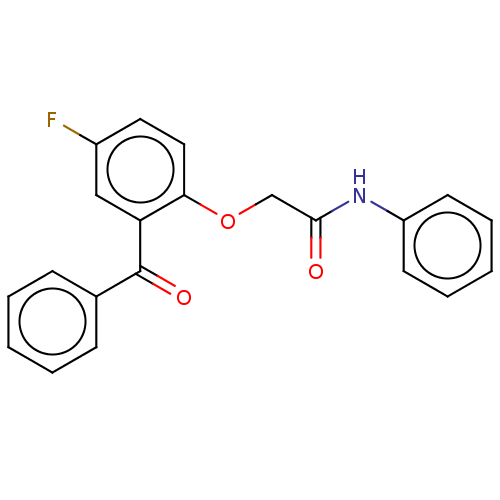 (CHEMBL46745) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470155 (CHEMBL288971) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470136 (CHEMBL296939 | GF-128590) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470145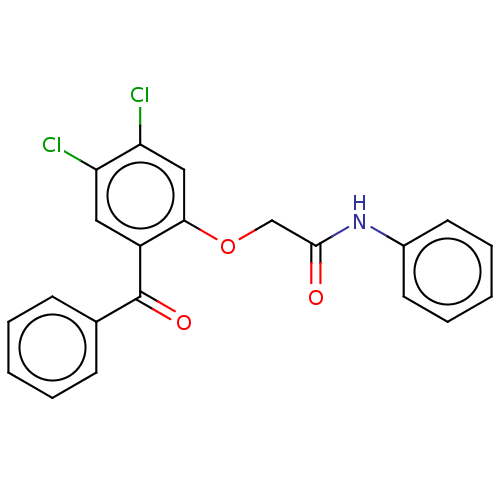 (CHEMBL46925) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470146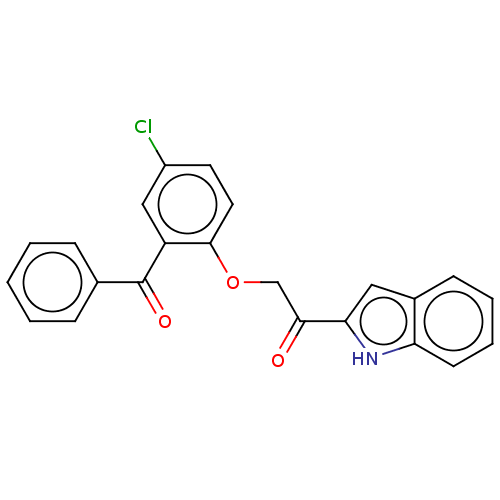 (CHEMBL417183) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470138 (CHEMBL46650) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470151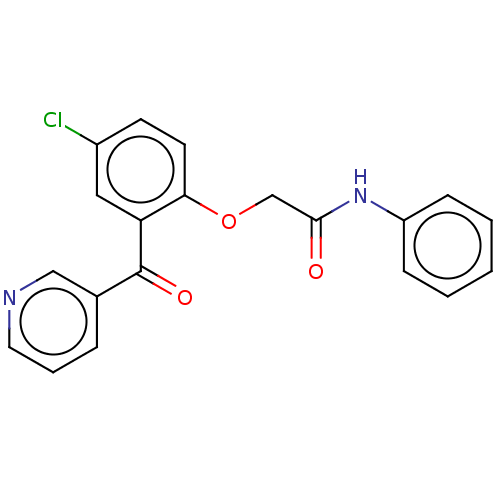 (CHEMBL297010) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 436 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470144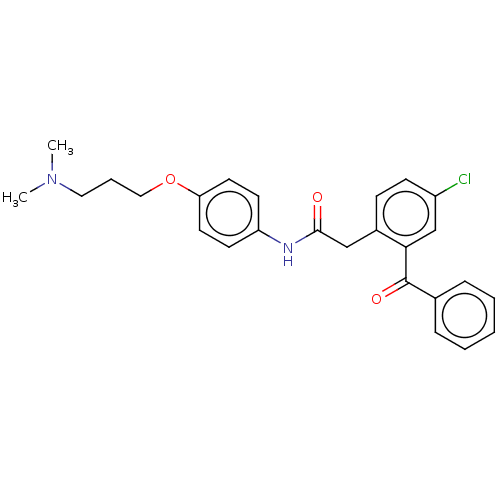 (CHEMBL43263) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470148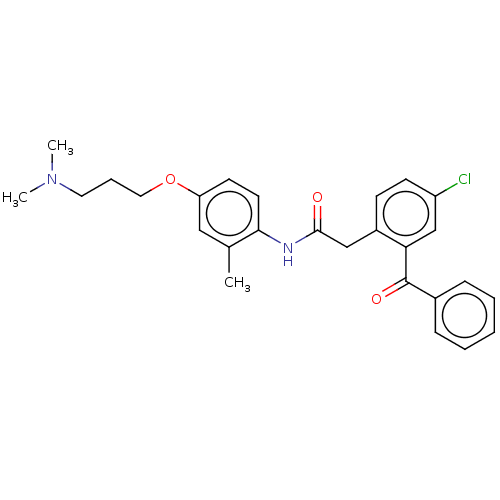 (CHEMBL42078) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 752 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470137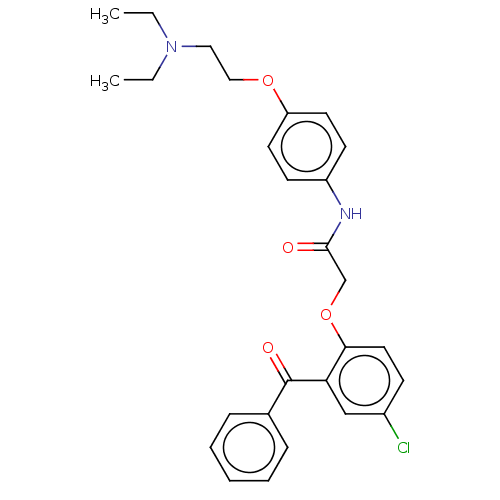 (CHEMBL45100) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470166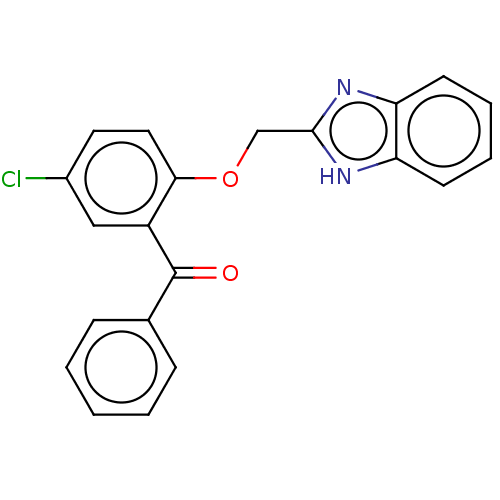 (CHEMBL44002) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470140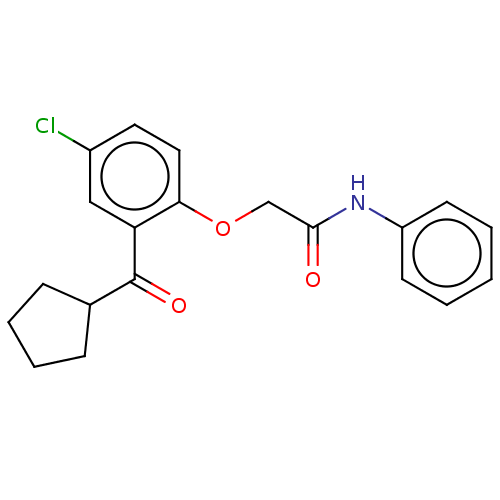 (CHEMBL42066) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470152 (CHEMBL42823) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470168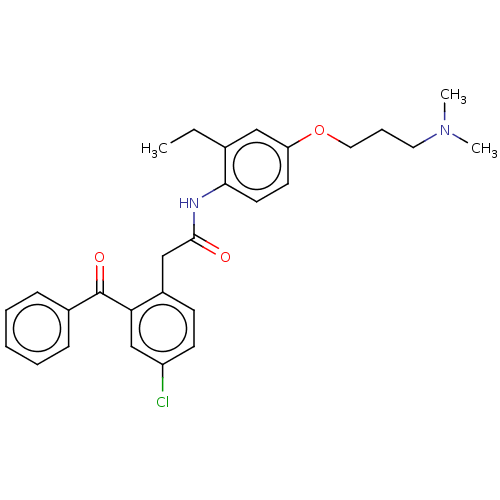 (CHEMBL46977) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470142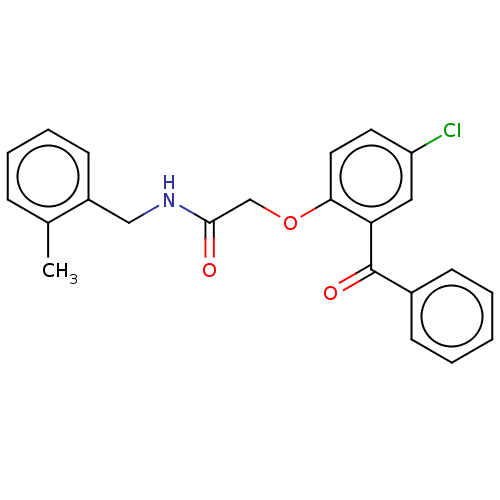 (CHEMBL46675) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470154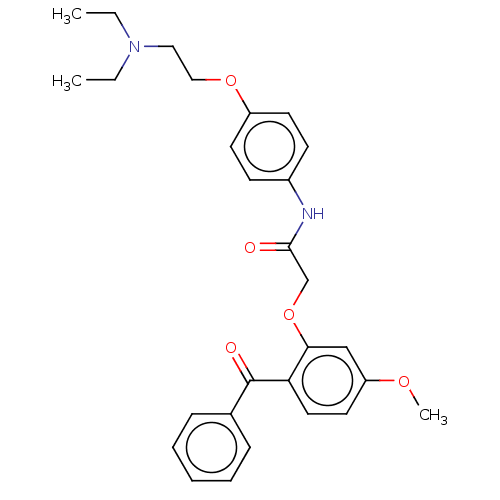 (CHEMBL47214) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470153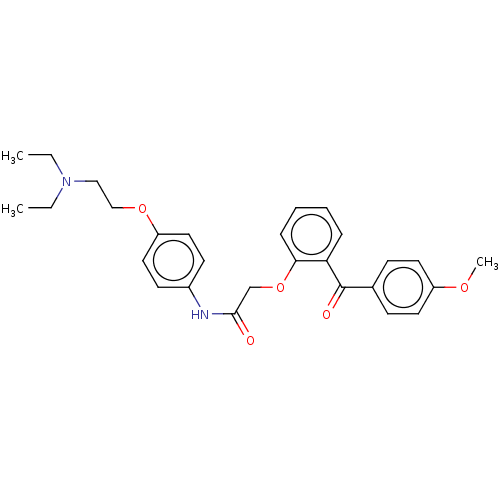 (CHEMBL415908) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470143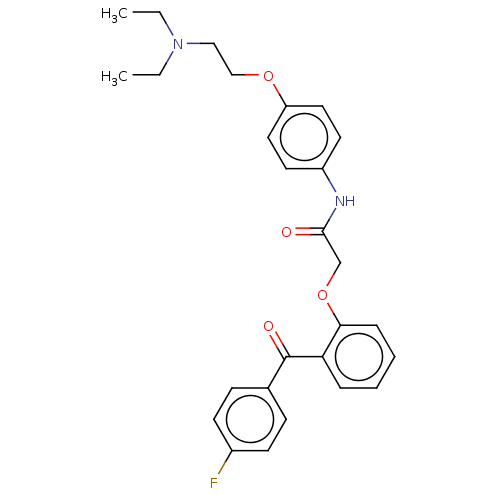 (CHEMBL295590) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470135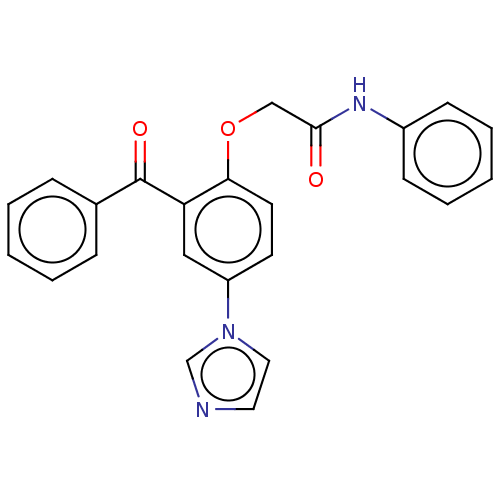 (CHEMBL289000) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470165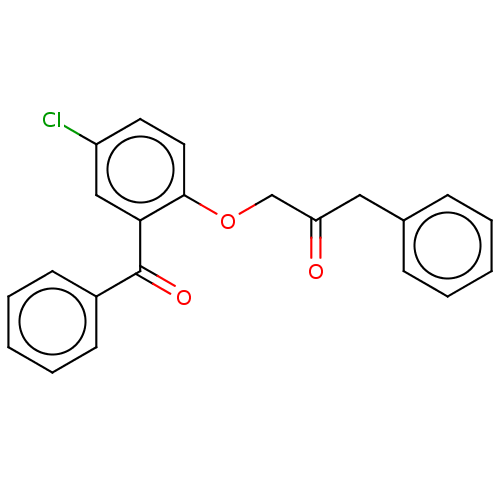 (CHEMBL441039) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470164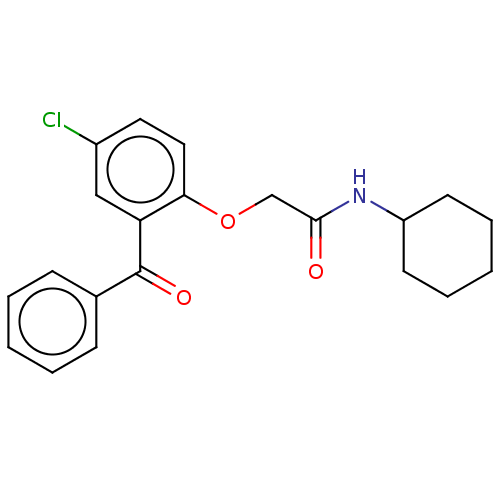 (CHEMBL298060) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470147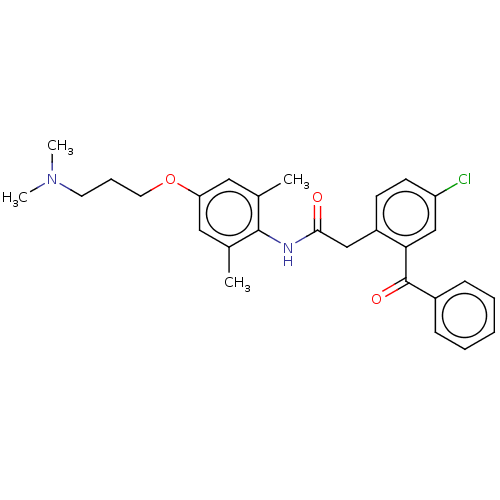 (CHEMBL44592) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470163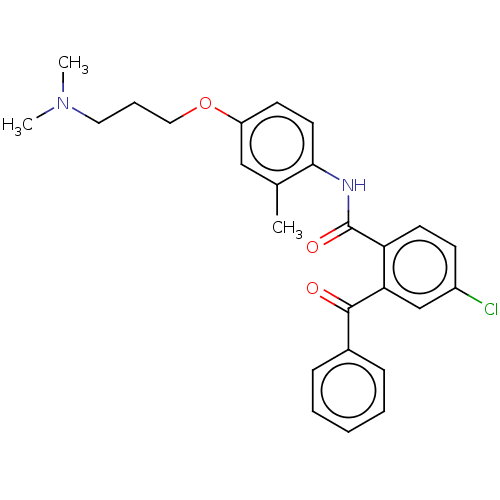 (CHEMBL46627) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470167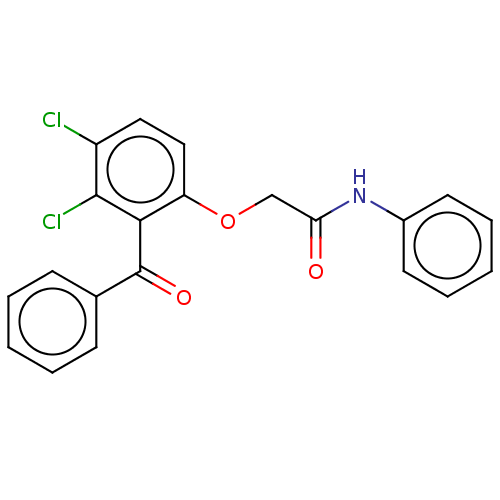 (CHEMBL44845) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470150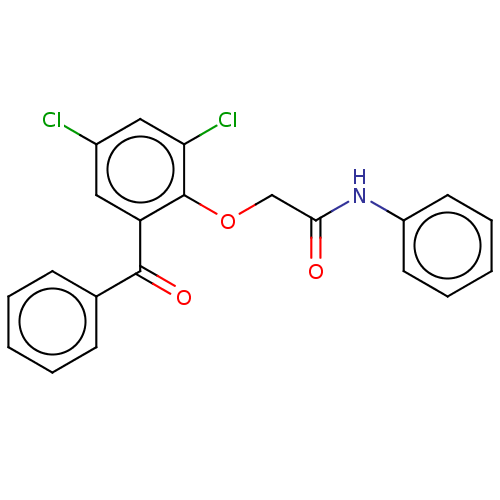 (CHEMBL46446) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470141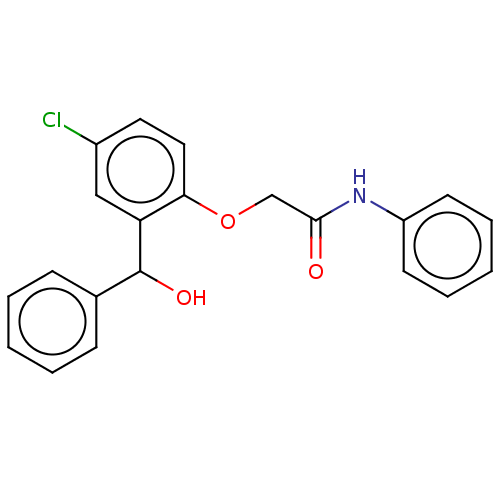 (CHEMBL295678) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470134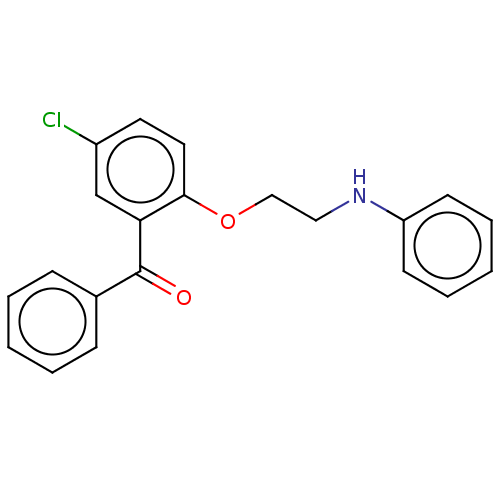 (CHEMBL46865) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50470149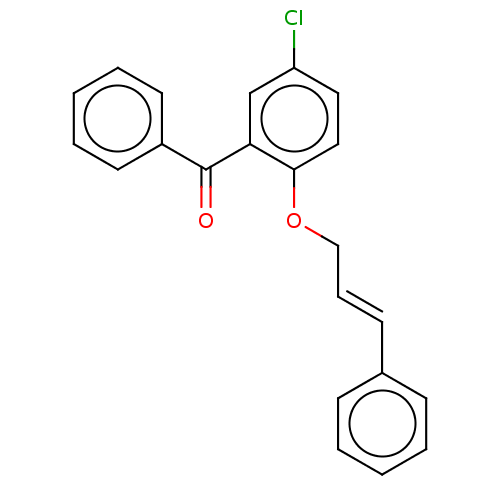 (CHEMBL46981) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 38: 1657-65 (1995) Article DOI: 10.1021/jm00010a010 BindingDB Entry DOI: 10.7270/Q27947DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||