 Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50007001
Found 48 hits Enz. Inhib. hit(s) with all data for entry = 50007001 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279

(CHEMBL4436207)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)B(O)O |r| Show InChI InChI=1S/C27H37BN2O9S/c1-18(2)15-30(40(35,36)21-10-8-20(9-11-21)28(33)34)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)39-25-17-38-26-22(25)12-13-37-26/h3-11,18,22-26,31,33-34H,12-17H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737
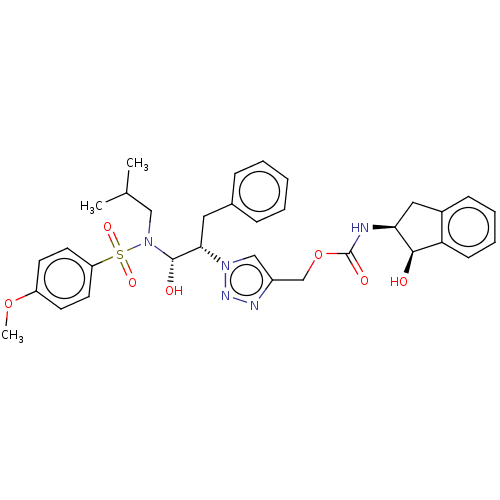
(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750
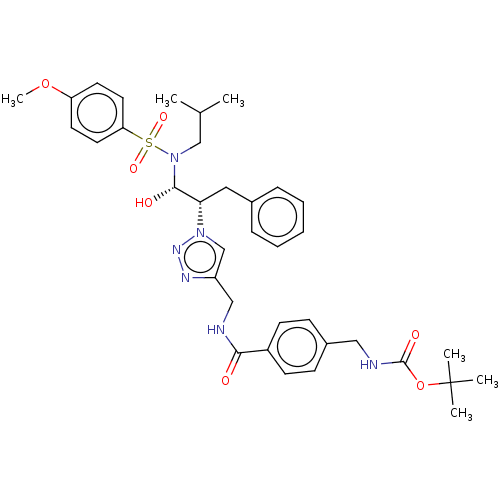
(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50505281
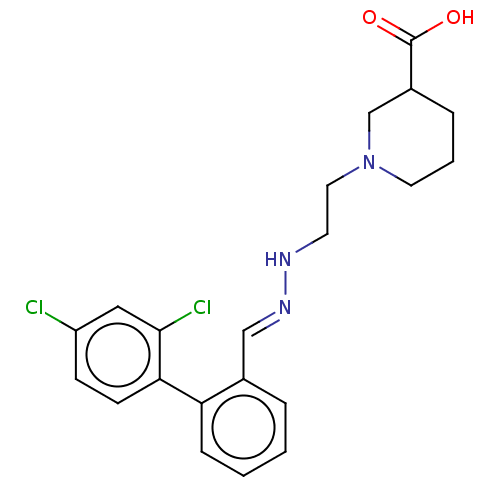
(CHEMBL2315948)Show SMILES OC(=O)C1CCCN(CCN\N=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 Show InChI InChI=1S/C21H23Cl2N3O2/c22-17-7-8-19(20(23)12-17)18-6-2-1-4-15(18)13-25-24-9-11-26-10-3-5-16(14-26)21(27)28/h1-2,4,6-8,12-13,16,24H,3,5,9-11,14H2,(H,27,28)/b25-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of NO711 binding to mouse GAT1 receptor expressed in HEK293 cell membranes incubated for 1 hr by LC-ESI-MS/MS analysis |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50505274
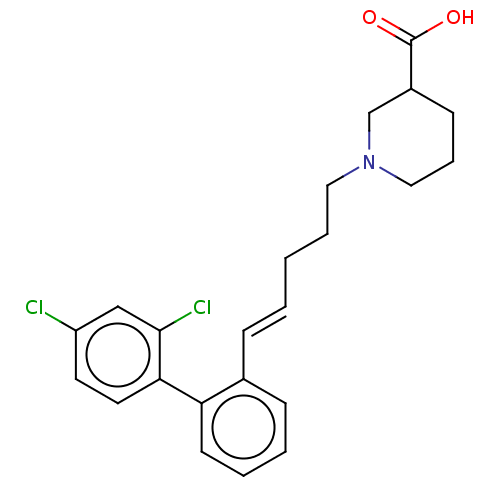
(CHEMBL2315639)Show SMILES OC(=O)C1CCCN(CCC\C=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 Show InChI InChI=1S/C23H25Cl2NO2/c24-19-11-12-21(22(25)15-19)20-10-4-3-8-17(20)7-2-1-5-13-26-14-6-9-18(16-26)23(27)28/h2-4,7-8,10-12,15,18H,1,5-6,9,13-14,16H2,(H,27,28)/b7-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of NO711 binding to mouse GAT1 receptor expressed in HEK293 cell membranes incubated for 1 hr by LC-ESI-MS/MS analysis |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 using GGEEEEYFELVKKKK substrate preincubated for 20 mis followed by addition of ATP and measured after 120 mins in presence ... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50505275
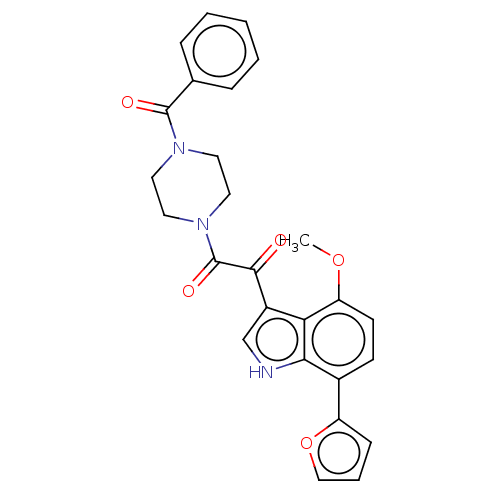
(CHEMBL4573432)Show SMILES COc1ccc(-c2ccco2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C26H23N3O5/c1-33-21-10-9-18(20-8-5-15-34-20)23-22(21)19(16-27-23)24(30)26(32)29-13-11-28(12-14-29)25(31)17-6-3-2-4-7-17/h2-10,15-16,27H,11-14H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 JRFL gp120 assessed as reduction in CD4/gp 120 complex formation incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50121210
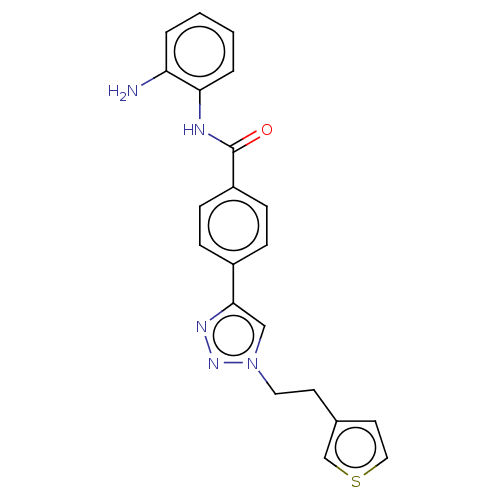
(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/GST-tagged NCOR1 DAD (397 to 503 residues) expressed in baculovirus expression system using Fluor de Lys as sub... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Nuclear receptor corepressor 1
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC3/GST-tagged NCOR1 DAD (397 to 503 residues) expressed in baculovirus expression system using Fluor de Lys as sub... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50505276
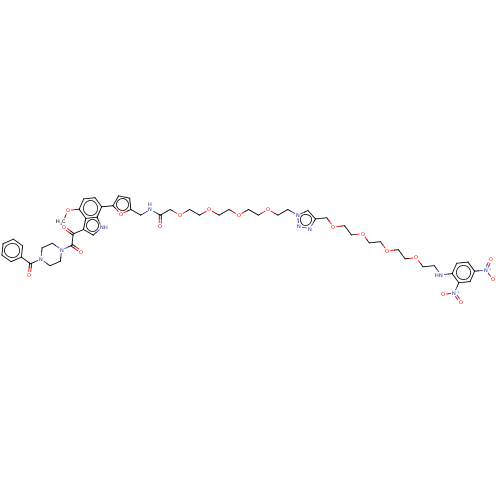
(CHEMBL4540823)Show SMILES COc1ccc(-c2ccc(CNC(=O)COCCOCCOCCOCCn3cc(COCCOCCOCCOCCNc4ccc(cc4[N+]([O-])=O)[N+]([O-])=O)nn3)o2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C54H66N10O18/c1-73-48-12-9-43(51-50(48)44(35-57-51)52(66)54(68)61-16-14-60(15-17-61)53(67)39-5-3-2-4-6-39)47-11-8-42(82-47)34-56-49(65)38-81-32-30-79-28-26-77-24-22-75-20-18-62-36-40(58-59-62)37-80-31-29-78-27-25-76-23-21-74-19-13-55-45-10-7-41(63(69)70)33-46(45)64(71)72/h2-12,33,35-36,55,57H,13-32,34,37-38H2,1H3,(H,56,65) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 JRFL gp120 assessed as reduction in CD4/gp 120 complex formation incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) substrate preincubated for 20 mis followed by addition of ATP and measured after 120 mins in prese... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 using poly[Glu:Tyr] (4:1) substrate preincubated for 20 mis followed by addition of ATP and measured after 120 mins in prese... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using KKSRGDYMTMQIG] substrate preincubated for 20 mis followed by addition of ATP and measured after 120 mins in presence o... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50133282
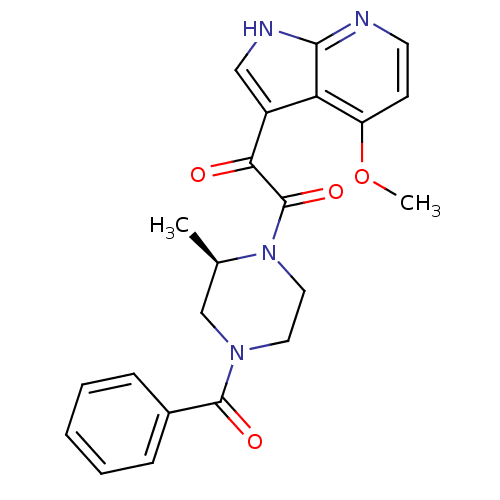
((R)-1-(4-benzoyl-2-methylpiperazin-1-yl)-2-(4-meth...)Show SMILES COc1ccnc2[nH]cc(C(=O)C(=O)N3CCN(C[C@H]3C)C(=O)c3ccccc3)c12 |r| Show InChI InChI=1S/C22H22N4O4/c1-14-13-25(21(28)15-6-4-3-5-7-15)10-11-26(14)22(29)19(27)16-12-24-20-18(16)17(30-2)8-9-23-20/h3-9,12,14H,10-11,13H2,1-2H3,(H,23,24)/t14-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 JRFL gp120 assessed as reduction in CD4/gp 120 complex formation incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal GST-tagged HDAC1 expressed in baculovirus Sf9 insect cells using HDAC Glo I/II substrate by Ki... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50505273
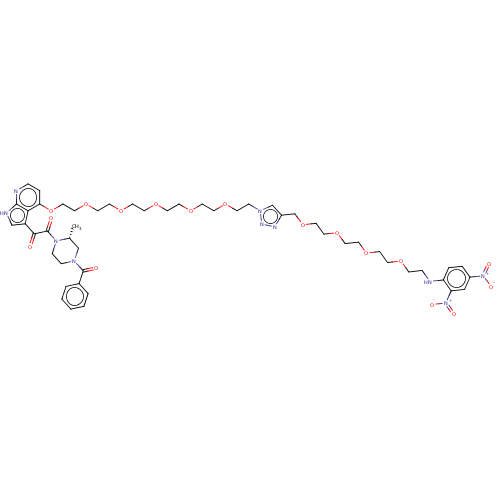
(CHEMBL3800574)Show SMILES C[C@@H]1CN(CCN1C(=O)C(=O)c1c[nH]c2nccc(OCCOCCOCCOCCOCCOCCn3cc(COCCOCCOCCOCCNc4ccc(cc4[N+]([O-])=O)[N+]([O-])=O)nn3)c12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C50H66N10O17/c1-38-35-56(49(62)39-5-3-2-4-6-39)12-13-58(38)50(63)47(61)42-34-53-48-46(42)45(9-10-52-48)77-32-31-75-28-27-73-24-23-72-22-21-71-20-18-69-16-14-57-36-40(54-55-57)37-76-30-29-74-26-25-70-19-17-68-15-11-51-43-8-7-41(59(64)65)33-44(43)60(66)67/h2-10,33-34,36,38,51H,11-32,35,37H2,1H3,(H,52,53)/t38-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 JRFL gp120 assessed as reduction in CD4/gp 120 complex formation incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399672

(CHEMBL2178342)Show InChI InChI=1S/C16H14N4O2S/c21-16(18-22)13-6-4-5-12(9-13)15-10-20(19-17-15)11-23-14-7-2-1-3-8-14/h1-10,22H,11H2,(H,18,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using Fluoro-Substrate Peptide as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal GST-tagged HDAC4 (627 to end residues) expressed in baculovirus Sf9 insect cells using HDAC cl... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC6 expressed in insect cells using Fluor de Lys as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC6 expressed in insect cells using Fluor de Lys as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal GST-tagged HDAC4 (627 to end residues) expressed in baculovirus Sf9 insect cells using HDAC cl... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC (unknown origin) using Fluoro-Substrate Peptide as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC (unknown origin) using Fluoro-Substrate Peptide as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) using Fluoro-Substrate Peptide as substrate by fluorescence assay |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal GST-tagged HDAC1 expressed in baculovirus Sf9 insect cells using HDAC Glo I/II substrate by Ki... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50505277
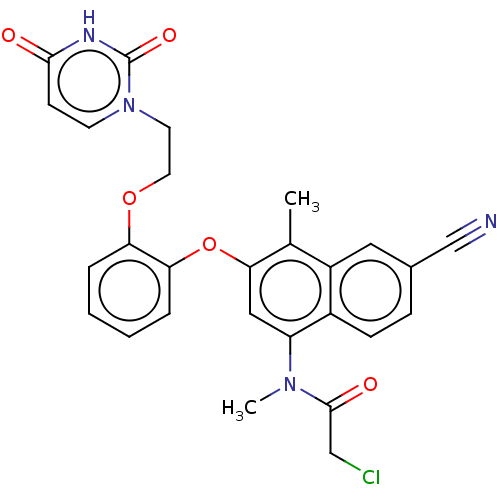
(CHEMBL4553027)Show SMILES CN(C(=O)CCl)c1cc(Oc2ccccc2OCCn2ccc(=O)[nH]c2=O)c(C)c2cc(ccc12)C#N Show InChI InChI=1S/C27H23ClN4O5/c1-17-20-13-18(16-29)7-8-19(20)21(31(2)26(34)15-28)14-24(17)37-23-6-4-3-5-22(23)36-12-11-32-10-9-25(33)30-27(32)35/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,33,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50505278
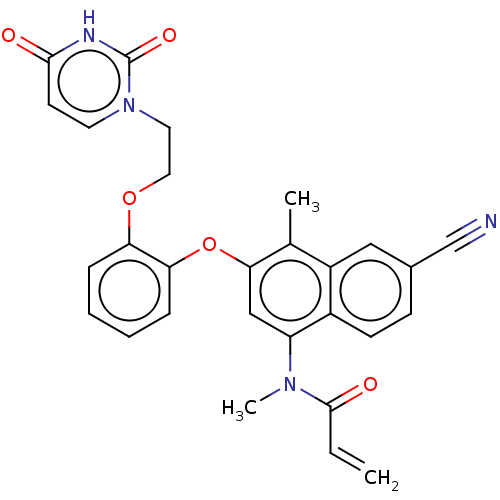
(CHEMBL4578515)Show SMILES CN(C(=O)C=C)c1cc(Oc2ccccc2OCCn2ccc(=O)[nH]c2=O)c(C)c2cc(ccc12)C#N Show InChI InChI=1S/C28H24N4O5/c1-4-27(34)31(3)22-16-25(18(2)21-15-19(17-29)9-10-20(21)22)37-24-8-6-5-7-23(24)36-14-13-32-12-11-26(33)30-28(32)35/h4-12,15-16H,1,13-14H2,2-3H3,(H,30,33,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50505280
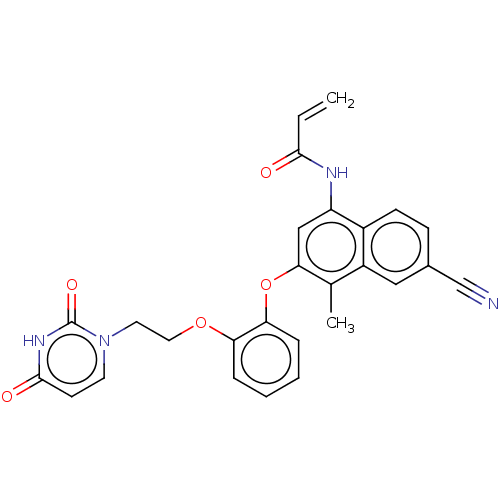
(CHEMBL4528039)Show SMILES Cc1c(Oc2ccccc2OCCn2ccc(=O)[nH]c2=O)cc(NC(=O)C=C)c2ccc(cc12)C#N Show InChI InChI=1S/C27H22N4O5/c1-3-25(32)29-21-15-24(17(2)20-14-18(16-28)8-9-19(20)21)36-23-7-5-4-6-22(23)35-13-12-31-11-10-26(33)30-27(31)34/h3-11,14-15H,1,12-13H2,2H3,(H,29,32)(H,30,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50505273

(CHEMBL3800574)Show SMILES C[C@@H]1CN(CCN1C(=O)C(=O)c1c[nH]c2nccc(OCCOCCOCCOCCOCCOCCn3cc(COCCOCCOCCOCCNc4ccc(cc4[N+]([O-])=O)[N+]([O-])=O)nn3)c12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C50H66N10O17/c1-38-35-56(49(62)39-5-3-2-4-6-39)12-13-58(38)50(63)47(61)42-34-53-48-46(42)45(9-10-52-48)77-32-31-75-28-27-73-24-23-72-22-21-71-20-18-69-16-14-57-36-40(54-55-57)37-76-30-29-74-26-25-70-19-17-68-15-11-51-43-8-7-41(59(64)65)33-44(43)60(66)67/h2-10,33-34,36,38,51H,11-32,35,37H2,1H3,(H,52,53)/t38-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 gp120 assessed as increase in anti-DNP binding to immobilized HIV-1 gp120 incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50505272
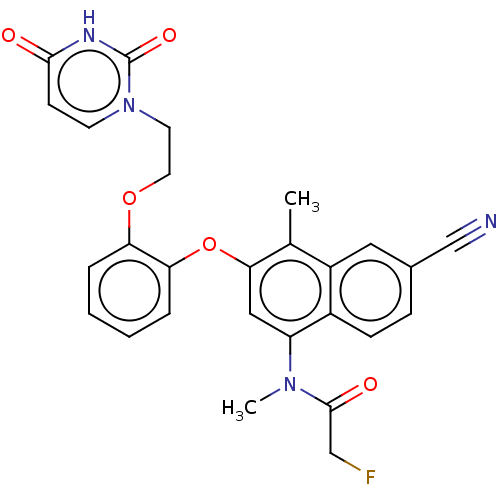
(CHEMBL4483362)Show SMILES CN(C(=O)CF)c1cc(Oc2ccccc2OCCn2ccc(=O)[nH]c2=O)c(C)c2cc(ccc12)C#N Show InChI InChI=1S/C27H23FN4O5/c1-17-20-13-18(16-29)7-8-19(20)21(31(2)26(34)15-28)14-24(17)37-23-6-4-3-5-22(23)36-12-11-32-10-9-25(33)30-27(32)35/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,33,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Envelope glycoprotein gp160
(Human immunodeficiency virus 1) | BDBM50505276

(CHEMBL4540823)Show SMILES COc1ccc(-c2ccc(CNC(=O)COCCOCCOCCOCCn3cc(COCCOCCOCCOCCNc4ccc(cc4[N+]([O-])=O)[N+]([O-])=O)nn3)o2)c2[nH]cc(C(=O)C(=O)N3CCN(CC3)C(=O)c3ccccc3)c12 Show InChI InChI=1S/C54H66N10O18/c1-73-48-12-9-43(51-50(48)44(35-57-51)52(66)54(68)61-16-14-60(15-17-61)53(67)39-5-3-2-4-6-39)47-11-8-42(82-47)34-56-49(65)38-81-32-30-79-28-26-77-24-22-75-20-18-62-36-40(58-59-62)37-80-31-29-78-27-25-76-23-21-74-19-13-55-45-10-7-41(63(69)70)33-46(45)64(71)72/h2-12,33,35-36,55,57H,13-32,34,37-38H2,1H3,(H,56,65) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 gp120 assessed as increase in anti-DNP binding to immobilized HIV-1 gp120 incubated for 1 hr by ELISA |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50501285
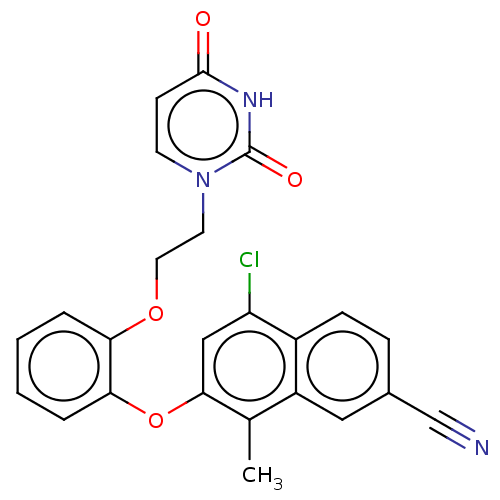
(CHEMBL3937452)Show SMILES Cc1c(Oc2ccccc2OCCn2ccc(=O)[nH]c2=O)cc(Cl)c2ccc(cc12)C#N Show InChI InChI=1S/C24H18ClN3O4/c1-15-18-12-16(14-26)6-7-17(18)19(25)13-22(15)32-21-5-3-2-4-20(21)31-11-10-28-9-8-23(29)27-24(28)30/h2-9,12-13H,10-11H2,1H3,(H,27,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo... |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data