 Found 66 hits Enz. Inhib. hit(s) with all data for entry = 50010918
Found 66 hits Enz. Inhib. hit(s) with all data for entry = 50010918 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543404
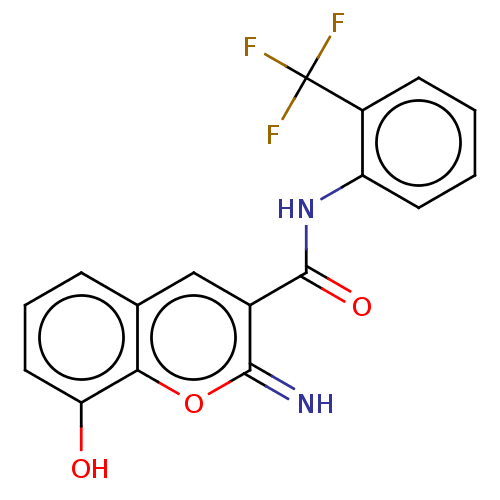
(CHEMBL4640154)Show SMILES Oc1cccc2cc(C(=O)Nc3ccccc3C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C17H11F3N2O3/c18-17(19,20)11-5-1-2-6-12(11)22-16(24)10-8-9-4-3-7-13(23)14(9)25-15(10)21/h1-8,21,23H,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543398
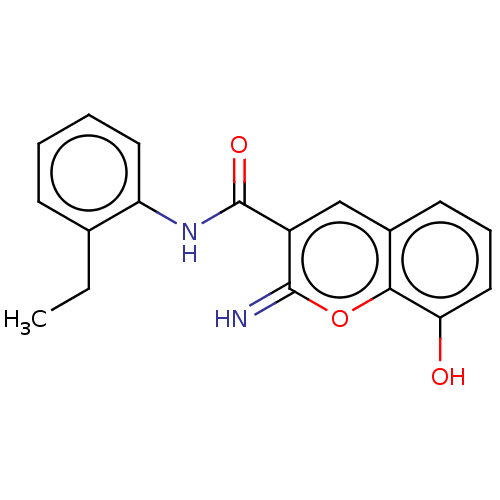
(CHEMBL4642187)Show InChI InChI=1S/C18H16N2O3/c1-2-11-6-3-4-8-14(11)20-18(22)13-10-12-7-5-9-15(21)16(12)23-17(13)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543409
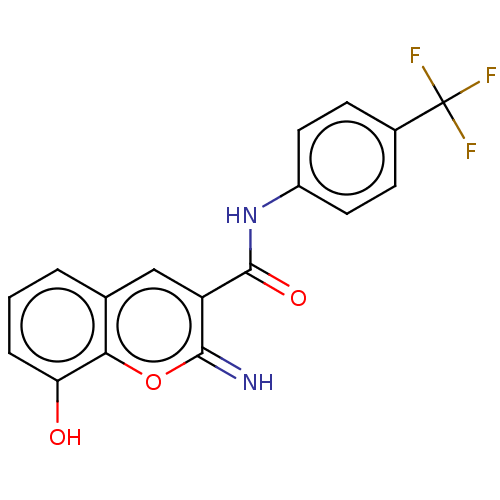
(CHEMBL4642852)Show SMILES Oc1cccc2cc(C(=O)Nc3ccc(cc3)C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C17H11F3N2O3/c18-17(19,20)10-4-6-11(7-5-10)22-16(24)12-8-9-2-1-3-13(23)14(9)25-15(12)21/h1-8,21,23H,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543401

(CHEMBL4644092)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543399
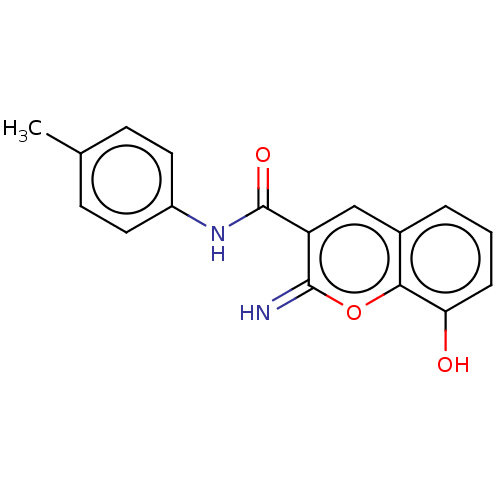
(CHEMBL4648793)Show InChI InChI=1S/C17H14N2O3/c1-10-5-7-12(8-6-10)19-17(21)13-9-11-3-2-4-14(20)15(11)22-16(13)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543402
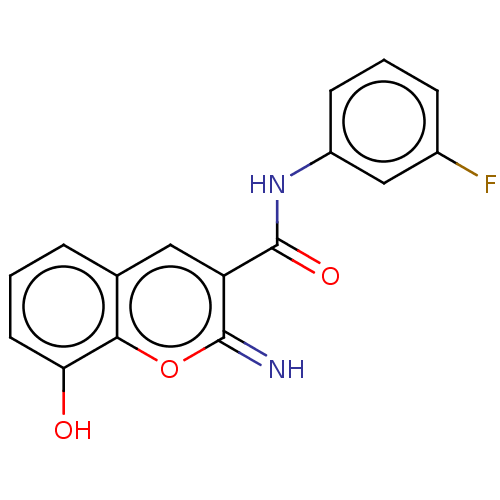
(CHEMBL4634960)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543407
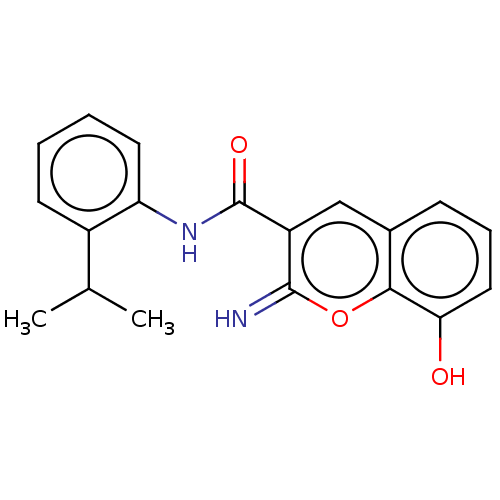
(CHEMBL4641899)Show InChI InChI=1S/C19H18N2O3/c1-11(2)13-7-3-4-8-15(13)21-19(23)14-10-12-6-5-9-16(22)17(12)24-18(14)20/h3-11,20,22H,1-2H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543397
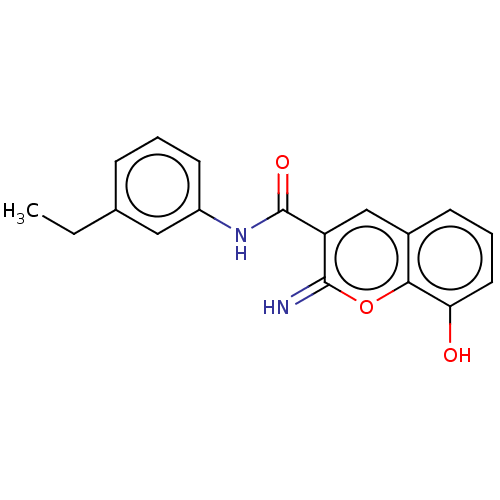
(CHEMBL4646593)Show InChI InChI=1S/C18H16N2O3/c1-2-11-5-3-7-13(9-11)20-18(22)14-10-12-6-4-8-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543400
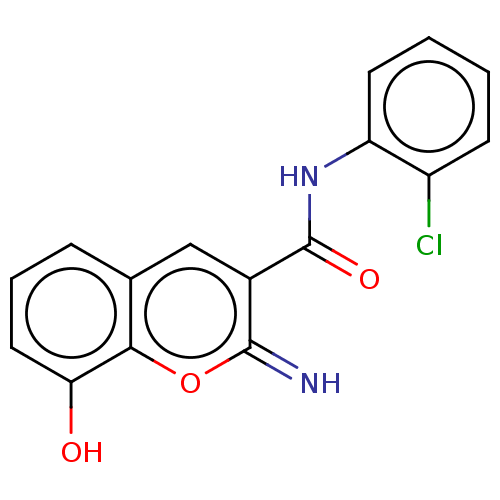
(CHEMBL4640248)Show InChI InChI=1S/C16H11ClN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543416
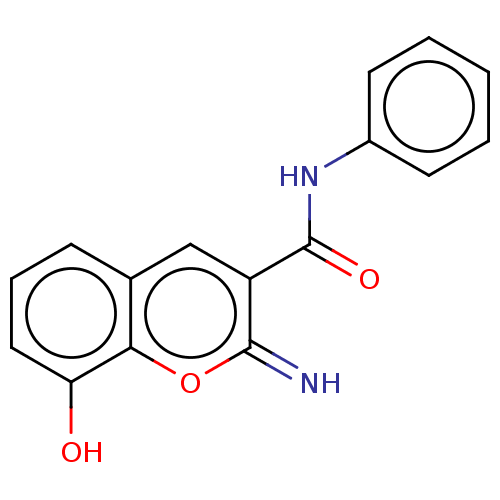
(CHEMBL4639909)Show InChI InChI=1S/C16H12N2O3/c17-15-12(16(20)18-11-6-2-1-3-7-11)9-10-5-4-8-13(19)14(10)21-15/h1-9,17,19H,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543396
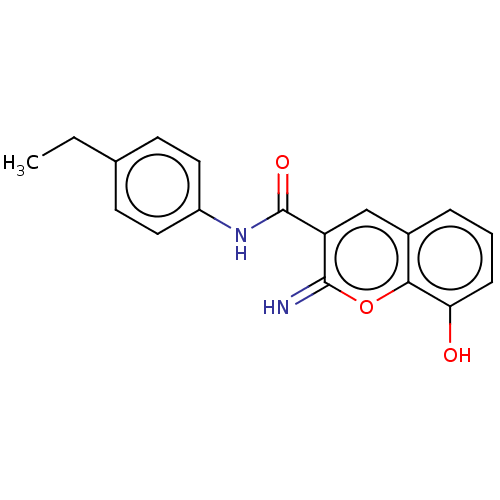
(CHEMBL4641012)Show InChI InChI=1S/C18H16N2O3/c1-2-11-6-8-13(9-7-11)20-18(22)14-10-12-4-3-5-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543405
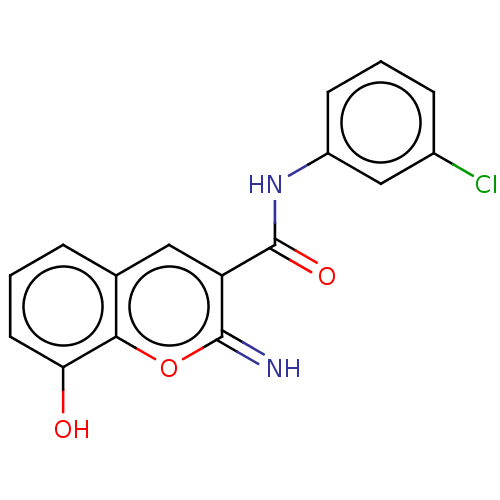
(CHEMBL4639417)Show InChI InChI=1S/C16H11ClN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543411
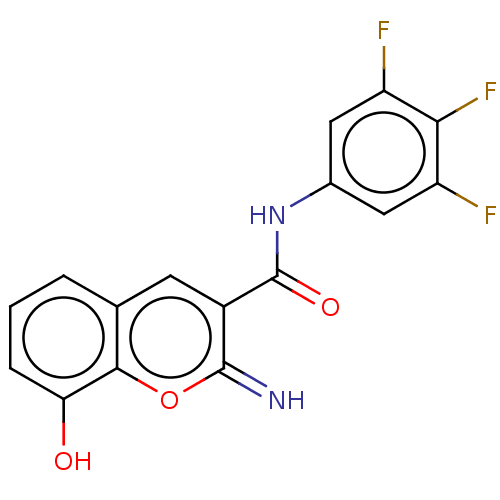
(CHEMBL4634140)Show SMILES Oc1cccc2cc(C(=O)Nc3cc(F)c(F)c(F)c3)c(=N)oc12 Show InChI InChI=1S/C16H9F3N2O3/c17-10-5-8(6-11(18)13(10)19)21-16(23)9-4-7-2-1-3-12(22)14(7)24-15(9)20/h1-6,20,22H,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543406

(CHEMBL4637597)Show InChI InChI=1S/C19H18N2O3/c1-11(2)12-5-3-7-14(9-12)21-19(23)15-10-13-6-4-8-16(22)17(13)24-18(15)20/h3-11,20,22H,1-2H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543410

(CHEMBL4633866)Show SMILES Oc1cccc2cc(C(=O)Nc3cccc(c3)C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C17H11F3N2O3/c18-17(19,20)10-4-2-5-11(8-10)22-16(24)12-7-9-3-1-6-13(23)14(9)25-15(12)21/h1-8,21,23H,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543414
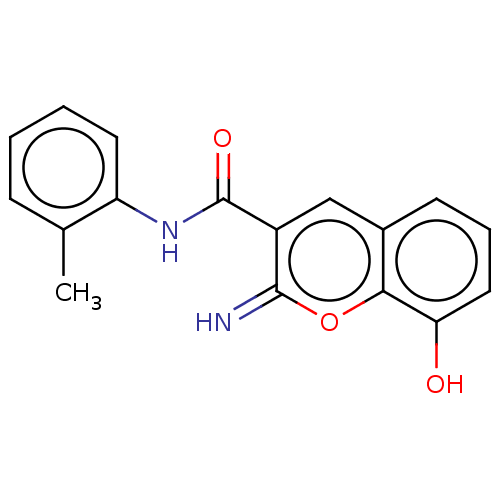
(CHEMBL4636345)Show InChI InChI=1S/C17H14N2O3/c1-10-5-2-3-7-13(10)19-17(21)12-9-11-6-4-8-14(20)15(11)22-16(12)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543415

(CHEMBL4641799)Show InChI InChI=1S/C16H11ClN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543413
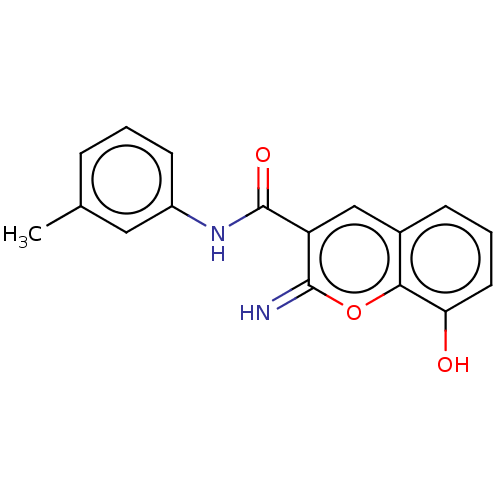
(CHEMBL4649502)Show InChI InChI=1S/C17H14N2O3/c1-10-4-2-6-12(8-10)19-17(21)13-9-11-5-3-7-14(20)15(11)22-16(13)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543400

(CHEMBL4640248)Show InChI InChI=1S/C16H11ClN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543408

(CHEMBL4640782)Show SMILES Oc1cccc2cc(C(=O)Nc3ccc(cc3C(F)(F)F)C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C18H10F6N2O3/c19-17(20,21)9-4-5-12(11(7-9)18(22,23)24)26-16(28)10-6-8-2-1-3-13(27)14(8)29-15(10)25/h1-7,25,27H,(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50213370
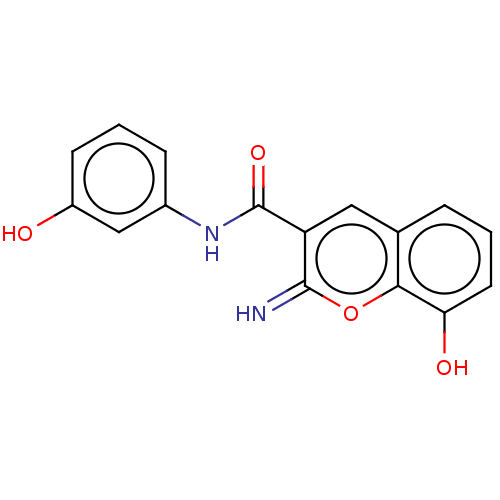
(CHEMBL82085)Show InChI InChI=1S/C16H12N2O4/c17-15-12(7-9-3-1-6-13(20)14(9)22-15)16(21)18-10-4-2-5-11(19)8-10/h1-8,17,19-20H,(H,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543403
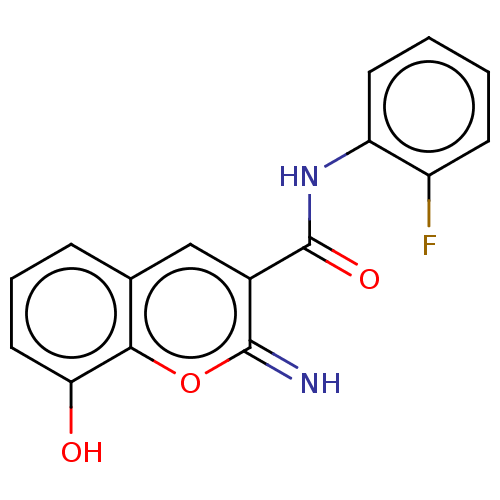
(CHEMBL4642678)Show InChI InChI=1S/C16H11FN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543404

(CHEMBL4640154)Show SMILES Oc1cccc2cc(C(=O)Nc3ccccc3C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C17H11F3N2O3/c18-17(19,20)11-5-1-2-6-12(11)22-16(24)10-8-9-4-3-7-13(23)14(9)25-15(10)21/h1-8,21,23H,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543405

(CHEMBL4639417)Show InChI InChI=1S/C16H11ClN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543412
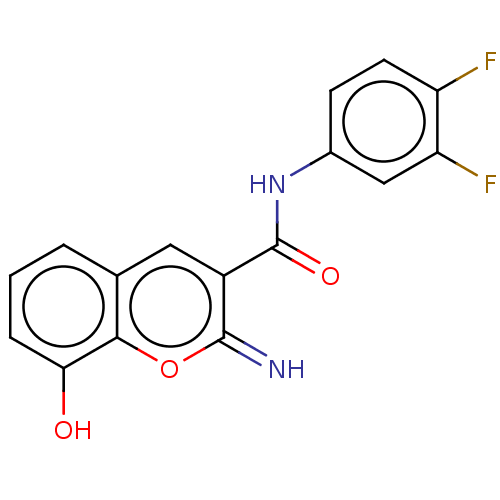
(CHEMBL4648687)Show InChI InChI=1S/C16H10F2N2O3/c17-11-5-4-9(7-12(11)18)20-16(22)10-6-8-2-1-3-13(21)14(8)23-15(10)19/h1-7,19,21H,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM35909
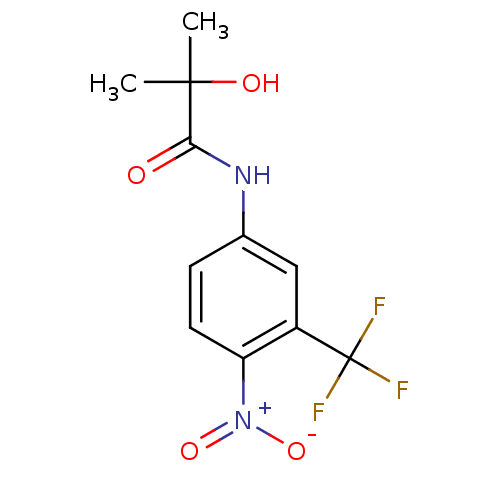
(2-Hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-ph...)Show SMILES CC(C)(O)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H11F3N2O4/c1-10(2,18)9(17)15-6-3-4-8(16(19)20)7(5-6)11(12,13)14/h3-5,18H,1-2H3,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543397

(CHEMBL4646593)Show InChI InChI=1S/C18H16N2O3/c1-2-11-5-3-7-13(9-11)20-18(22)14-10-12-6-4-8-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543399

(CHEMBL4648793)Show InChI InChI=1S/C17H14N2O3/c1-10-5-7-12(8-6-10)19-17(21)13-9-11-3-2-4-14(20)15(11)22-16(13)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50543420
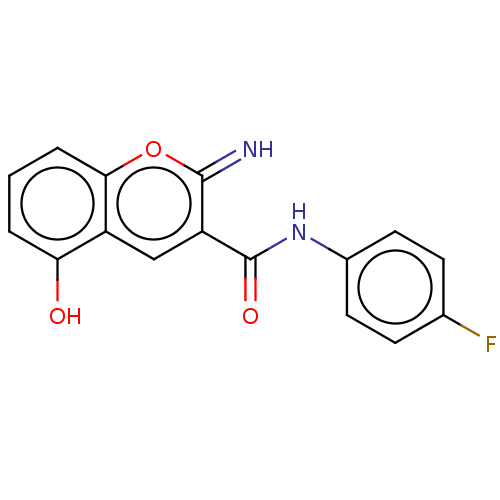
(CHEMBL4638008)Show InChI InChI=1S/C16H11FN2O3/c17-9-4-6-10(7-5-9)19-16(21)12-8-11-13(20)2-1-3-14(11)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543402

(CHEMBL4634960)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Carbonyl reductase [NADPH] 1
(Homo sapiens (Human)) | BDBM50543401

(CHEMBL4644092)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CBR1 (unknown origin) |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543399

(CHEMBL4648793)Show InChI InChI=1S/C17H14N2O3/c1-10-5-7-12(8-6-10)19-17(21)13-9-11-3-2-4-14(20)15(11)22-16(13)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543402

(CHEMBL4634960)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543400

(CHEMBL4640248)Show InChI InChI=1S/C16H11ClN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543396

(CHEMBL4641012)Show InChI InChI=1S/C18H16N2O3/c1-2-11-6-8-13(9-7-11)20-18(22)14-10-12-4-3-5-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543403

(CHEMBL4642678)Show InChI InChI=1S/C16H11FN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50382163
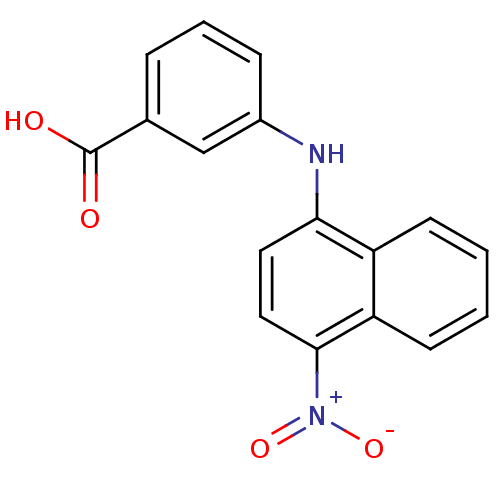
(CHEMBL2023820)Show SMILES OC(=O)c1cccc(Nc2ccc([N+]([O-])=O)c3ccccc23)c1 Show InChI InChI=1S/C17H12N2O4/c20-17(21)11-4-3-5-12(10-11)18-15-8-9-16(19(22)23)14-7-2-1-6-13(14)15/h1-10,18H,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543397

(CHEMBL4646593)Show InChI InChI=1S/C18H16N2O3/c1-2-11-5-3-7-13(9-11)20-18(22)14-10-12-6-4-8-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543401

(CHEMBL4644092)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543401

(CHEMBL4644092)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50543398

(CHEMBL4642187)Show InChI InChI=1S/C18H16N2O3/c1-2-11-6-3-4-8-14(11)20-18(22)13-10-12-7-5-9-15(21)16(12)23-17(13)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50543401

(CHEMBL4644092)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-6-11(7-5-10)19-16(21)12-8-9-2-1-3-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C2 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50543402

(CHEMBL4634960)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C2 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543404

(CHEMBL4640154)Show SMILES Oc1cccc2cc(C(=O)Nc3ccccc3C(F)(F)F)c(=N)oc12 Show InChI InChI=1S/C17H11F3N2O3/c18-17(19,20)11-5-1-2-6-12(11)22-16(24)10-8-9-4-3-7-13(23)14(9)25-15(10)21/h1-8,21,23H,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543397

(CHEMBL4646593)Show InChI InChI=1S/C18H16N2O3/c1-2-11-5-3-7-13(9-11)20-18(22)14-10-12-6-4-8-15(21)16(12)23-17(14)19/h3-10,19,21H,2H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543399

(CHEMBL4648793)Show InChI InChI=1S/C17H14N2O3/c1-10-5-7-12(8-6-10)19-17(21)13-9-11-3-2-4-14(20)15(11)22-16(13)18/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543405

(CHEMBL4639417)Show InChI InChI=1S/C16H11ClN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543400

(CHEMBL4640248)Show InChI InChI=1S/C16H11ClN2O3/c17-11-5-1-2-6-12(11)19-16(21)10-8-9-4-3-7-13(20)14(9)22-15(10)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50543402

(CHEMBL4634960)Show InChI InChI=1S/C16H11FN2O3/c17-10-4-2-5-11(8-10)19-16(21)12-7-9-3-1-6-13(20)14(9)22-15(12)18/h1-8,18,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50213408
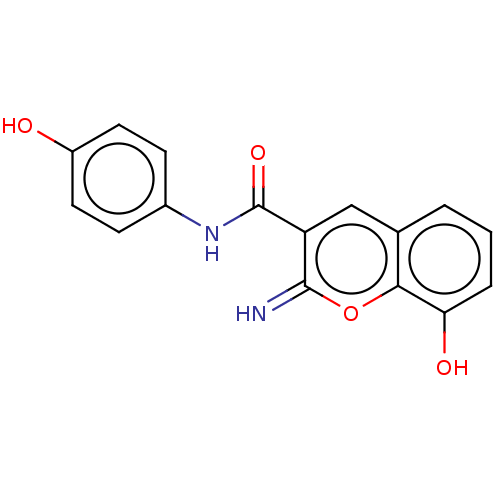
(CHEMBL79581)Show InChI InChI=1S/C16H12N2O4/c17-15-12(8-9-2-1-3-13(20)14(9)22-15)16(21)18-10-4-6-11(19)7-5-10/h1-8,17,19-20H,(H,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level |
J Med Chem 63: 10396-10411 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00939
BindingDB Entry DOI: 10.7270/Q2V40ZSN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data