 Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50016957
Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50016957 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50577605
(CHEMBL4876917) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50577613

(CHEMBL4857804) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50506744
(CHEBI:73038 | Parecoxib | SC-69124)Show SMILES CCC(=O)NS(=O)(=O)c1ccc(cc1)-c1c(C)onc1-c1ccccc1 Show InChI InChI=1S/C19H18N2O4S/c1-3-17(22)21-26(23,24)16-11-9-14(10-12-16)18-13(2)25-20-19(18)15-7-5-4-6-8-15/h4-12H,3H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50002861
(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50250885

(CHEBI:65019 | CHEMBL2270066)Show InChI InChI=1S/C10H9NO2/c1-13-10(12)8-6-11-9-5-3-2-4-7(8)9/h2-6,11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50101853
((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50318491
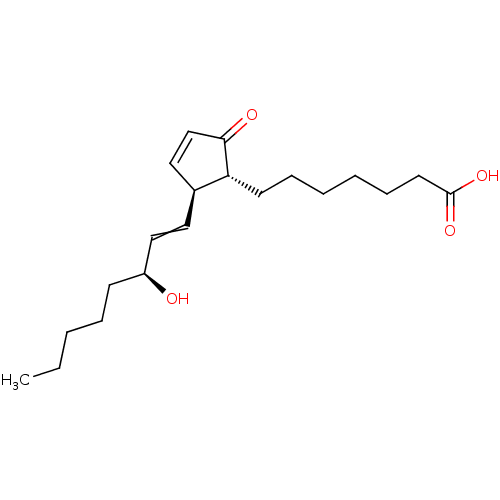
(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50318491

(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Rattus norvegicus) | BDBM50318491

(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Rattus norvegicus) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Rattus norvegicus) | BDBM50318491

(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Rattus norvegicus) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM22985
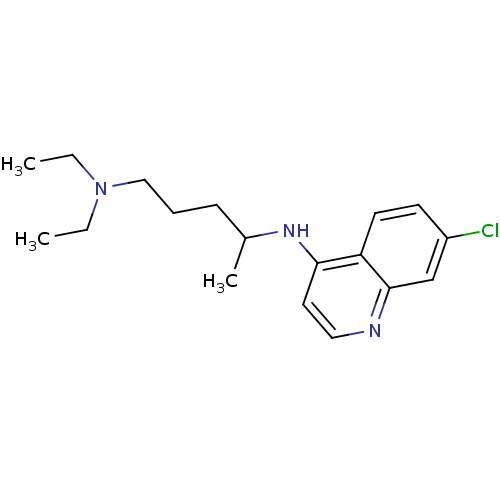
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM23300
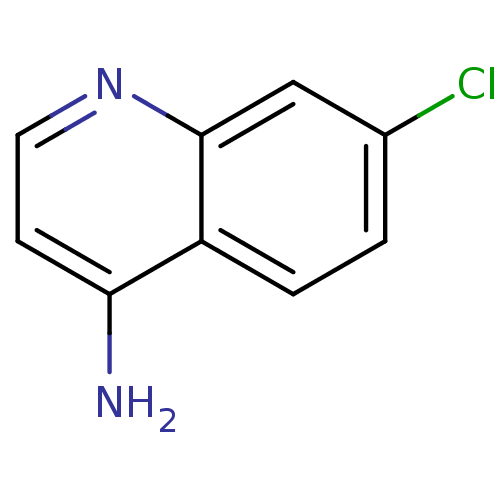
(4-amino-7-chloroquinoline (ACQ)-based compound, 5 ...)Show InChI InChI=1S/C9H7ClN2/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.59E+5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50571370
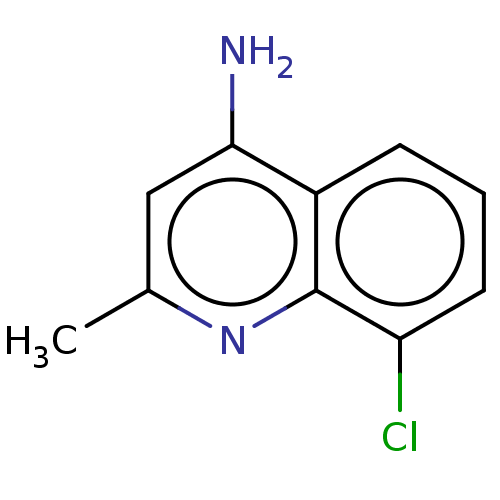
(CHEMBL4848185) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50294169

(5-chloro-1H-indole | CHEMBL555013)Show InChI InChI=1S/C8H6ClN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50358747
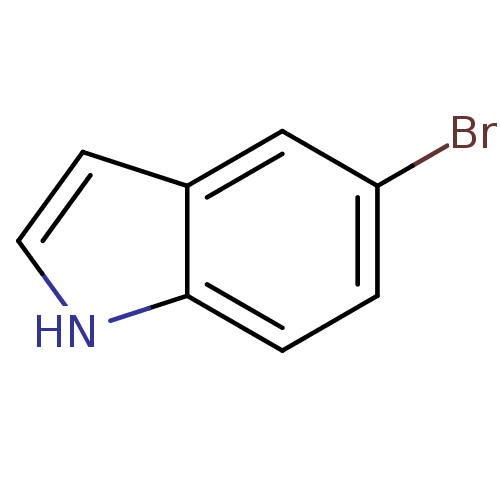
(CHEMBL325917)Show InChI InChI=1S/C8H6BrN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50598626
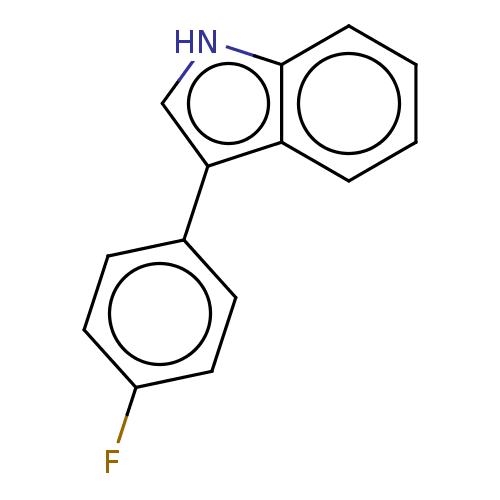
(CHEMBL5178735) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM86704
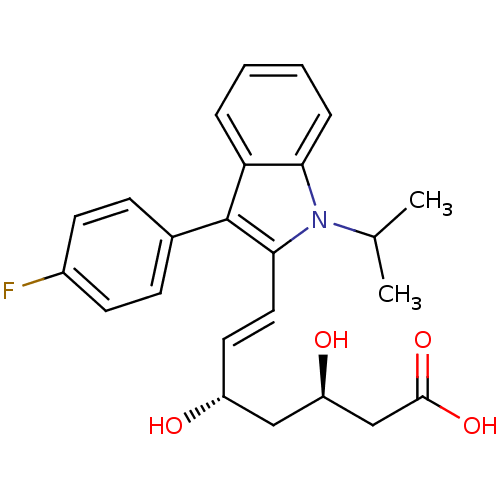
(CAS_93957-54-1 | Fluvastatin | cid_446155)Show SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |r| Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM86707
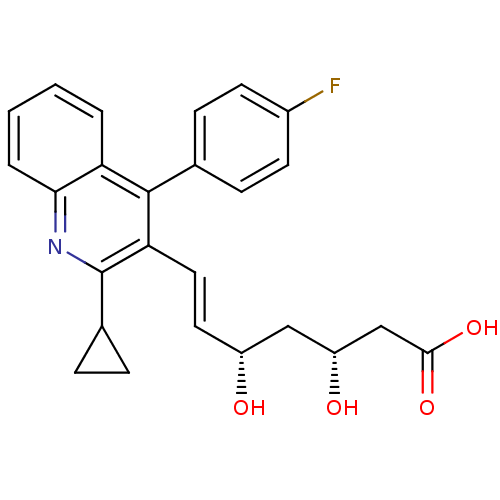
(CAS_147511-69-1 | Pitavastatin)Show SMILES O[C@H](C[C@H](O)\C=C\c1c(nc2ccccc2c1-c1ccc(F)cc1)C1CC1)CC(O)=O |r| Show InChI InChI=1S/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50318491

(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50318491

(7-((1R,2S)-2-((S)-3-hydroxyoct-1-enyl)-5-oxocyclop...)Show SMILES CCCCC[C@H](O)C=C[C@H]1C=CC(=O)[C@@H]1CCCCCCC(O)=O |r,c:10| Show InChI InChI=1S/C20H32O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12-18,21H,2-11H2,1H3,(H,23,24)/t16-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50150484
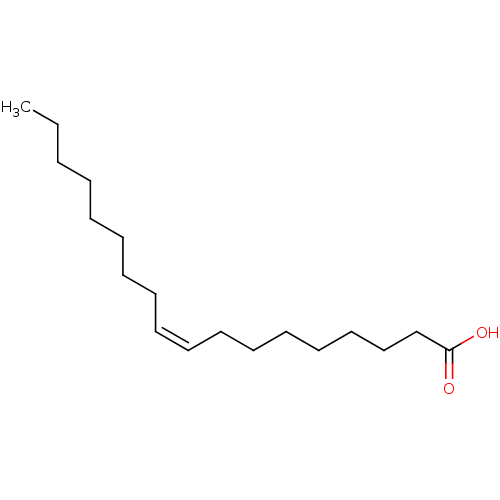
((Z)-9-octadecenoic acid | (Z)-Octadec-9-enoic acid...)Show InChI InChI=1S/C18H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H,19,20)/b10-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM22319
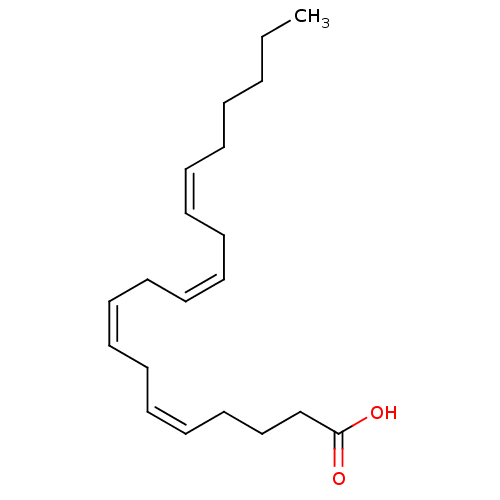
((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | ...)Show InChI InChI=1S/C20H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-,16-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM22231
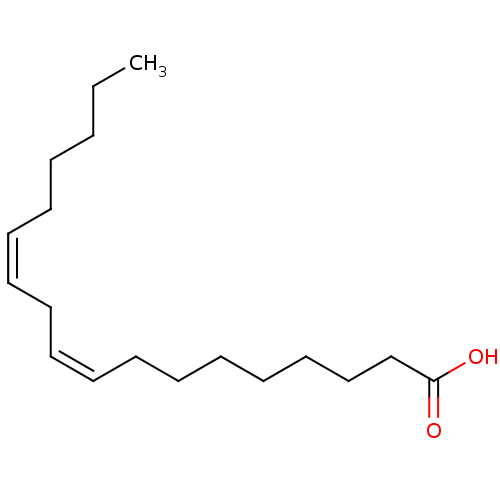
((9Z,12Z)-octadeca-9,12-dienoic acid | CHEMBL267476...)Show InChI InChI=1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h6-7,9-10H,2-5,8,11-17H2,1H3,(H,19,20)/b7-6-,10-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00585
BindingDB Entry DOI: 10.7270/Q24T6PD2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data