 Found 12 hits Enz. Inhib. hit(s) with all data for entry = 50017350
Found 12 hits Enz. Inhib. hit(s) with all data for entry = 50017350 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031612
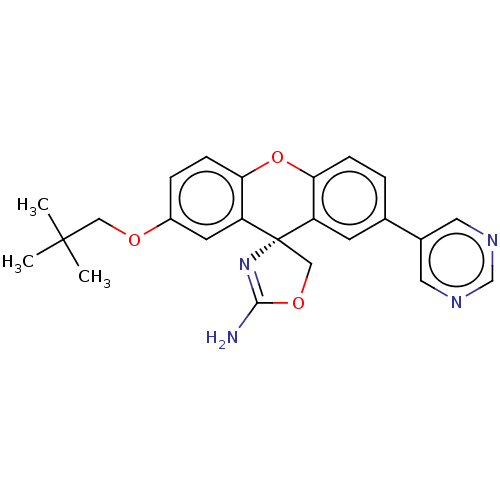
(CHEMBL3354688)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H24N4O3/c1-23(2,3)12-29-17-5-7-21-19(9-17)24(13-30-22(25)28-24)18-8-15(4-6-20(18)31-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50601401

(CHEMBL5201184)Show SMILES [H][C@]12CC[C@@H](C[C@]1([H])C1(CSC(N)=N1)c1cc(ccc1O2)-c1cncc(F)c1)NC(=O)C(C)(C)C |r,c:13| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50451624

(CHEMBL331897)Show SMILES CC[N+]1(C\C2=C\CCCCCC2)CCC(CC1)NC(=O)C1c2cc(Cl)ccc2Oc2ccc(Cl)cc12 |t:4| Show InChI InChI=1S/C30H36Cl2N2O2/c1-2-34(20-21-8-6-4-3-5-7-9-21)16-14-24(15-17-34)33-30(35)29-25-18-22(31)10-12-27(25)36-28-13-11-23(32)19-26(28)29/h8,10-13,18-19,24,29H,2-7,9,14-17,20H2,1H3/p+1/b21-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50031612

(CHEMBL3354688)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H24N4O3/c1-23(2,3)12-29-17-5-7-21-19(9-17)24(13-30-22(25)28-24)18-8-15(4-6-20(18)31-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098641
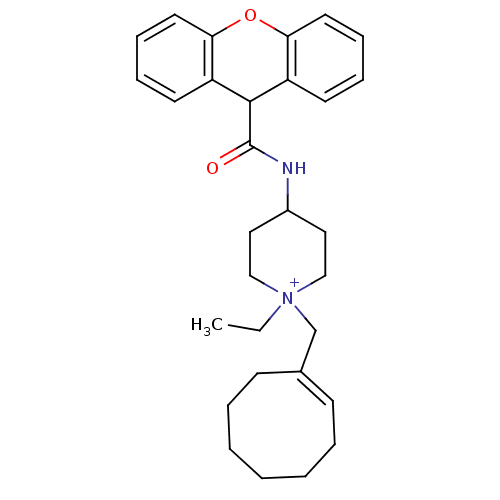
(1-Cyclooct-1-enylmethyl-1-ethyl-4-[(9H-xanthene-9-...)Show SMILES CC[N+]1(C\C2=C\CCCCCC2)CCC(CC1)NC(=O)C1c2ccccc2Oc2ccccc12 |t:4,(7.65,-27.47,;6.33,-28.27,;6.35,-29.81,;7.68,-30.58,;9.25,-29.88,;10.66,-30.48,;12.1,-29.88,;12.68,-28.47,;12.1,-27.02,;10.66,-26.45,;9.25,-27.05,;8.66,-28.47,;5,-29.05,;3.67,-29.81,;3.57,-31.55,;5.04,-32.12,;6.38,-31.32,;2.22,-32.32,;1.03,-31.32,;1.01,-29.81,;-.31,-32.12,;-.31,-33.66,;1.01,-34.44,;1.01,-35.97,;-.34,-36.71,;-1.66,-35.94,;-1.66,-34.43,;-3,-33.66,;-2.97,-32.12,;-4.29,-31.36,;-4.32,-29.81,;-2.97,-29.04,;-1.63,-29.81,;-1.63,-31.32,)| Show InChI InChI=1S/C30H38N2O2/c1-2-32(22-23-12-6-4-3-5-7-13-23)20-18-24(19-21-32)31-30(33)29-25-14-8-10-16-27(25)34-28-17-11-9-15-26(28)29/h8-12,14-17,24,29H,2-7,13,18-22H2,1H3/p+1/b23-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098634
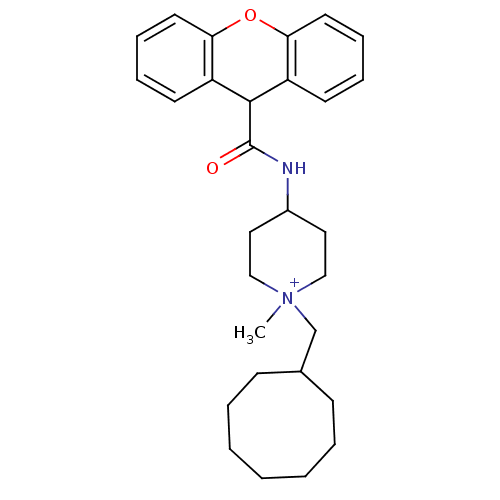
(1-Cyclooctylmethyl-1-methyl-4-[(9H-xanthene-9-carb...)Show SMILES C[N+]1(CC2CCCCCCC2)CCC(CC1)NC(=O)C1c2ccccc2Oc2ccccc12 |(5.5,2.52,;5.66,.6,;7.01,-.17,;8.57,.51,;7.97,1.93,;8.57,3.36,;9.99,3.95,;11.4,3.36,;12,1.95,;11.4,.51,;9.99,-.08,;4.31,1.35,;2.99,.6,;2.99,-.94,;4.34,-1.71,;5.69,-.94,;1.68,-1.73,;.36,-.96,;.33,.58,;-.98,-1.73,;-.98,-3.27,;.33,-4.05,;.33,-5.58,;-1.02,-6.35,;-2.33,-5.56,;-2.33,-4.04,;-3.65,-3.27,;-3.65,-1.73,;-4.96,-.97,;-4.99,.58,;-3.65,1.35,;-2.3,.58,;-2.3,-.96,)| Show InChI InChI=1S/C29H38N2O2/c1-31(21-22-11-5-3-2-4-6-12-22)19-17-23(18-20-31)30-29(32)28-24-13-7-9-15-26(24)33-27-16-10-8-14-25(27)28/h7-10,13-16,22-23,28H,2-6,11-12,17-21H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098650
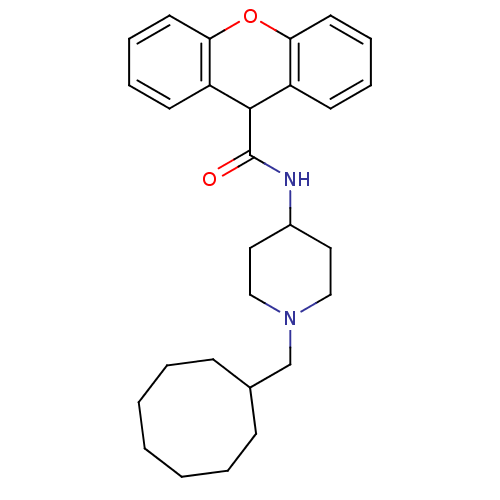
(9H-Xanthene-9-carboxylic acid (1-cyclooctylmethyl-...)Show SMILES O=C(NC1CCN(CC2CCCCCCC2)CC1)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C28H36N2O2/c31-28(27-23-12-6-8-14-25(23)32-26-15-9-7-13-24(26)27)29-22-16-18-30(19-17-22)20-21-10-4-2-1-3-5-11-21/h6-9,12-15,21-22,27H,1-5,10-11,16-20H2,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098629
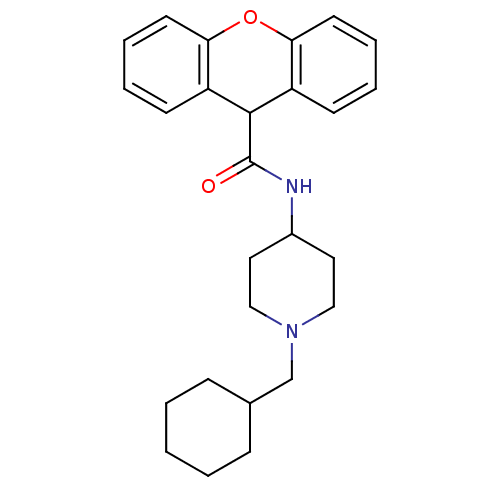
(9H-Xanthene-9-carboxylic acid (1-cyclohexylmethyl-...)Show SMILES O=C(NC1CCN(CC2CCCCC2)CC1)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C26H32N2O2/c29-26(27-20-14-16-28(17-15-20)18-19-8-2-1-3-9-19)25-21-10-4-6-12-23(21)30-24-13-7-5-11-22(24)25/h4-7,10-13,19-20,25H,1-3,8-9,14-18H2,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098625
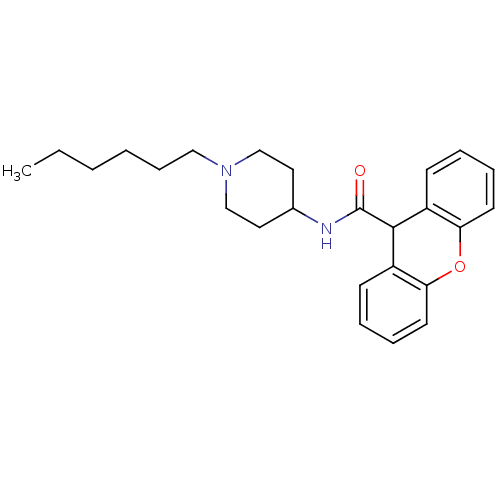
(9H-Xanthene-9-carboxylic acid (1-hexyl-piperidin-4...)Show InChI InChI=1S/C25H32N2O2/c1-2-3-4-9-16-27-17-14-19(15-18-27)26-25(28)24-20-10-5-7-12-22(20)29-23-13-8-6-11-21(23)24/h5-8,10-13,19,24H,2-4,9,14-18H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098647

(9H-Xanthene-9-carboxylic acid (1-cyclodecylmethyl-...)Show SMILES O=C(NC1CCN(CC2CCCCCCCCC2)CC1)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C30H40N2O2/c33-30(29-25-14-8-10-16-27(25)34-28-17-11-9-15-26(28)29)31-24-18-20-32(21-19-24)22-23-12-6-4-2-1-3-5-7-13-23/h8-11,14-17,23-24,29H,1-7,12-13,18-22H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098623

(9H-Xanthene-9-carboxylic acid (1-naphthalen-2-ylme...)Show SMILES O=C(NC1CCN(Cc2ccc3ccccc3c2)CC1)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C30H28N2O2/c33-30(29-25-9-3-5-11-27(25)34-28-12-6-4-10-26(28)29)31-24-15-17-32(18-16-24)20-21-13-14-22-7-1-2-8-23(22)19-21/h1-14,19,24,29H,15-18,20H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50098646
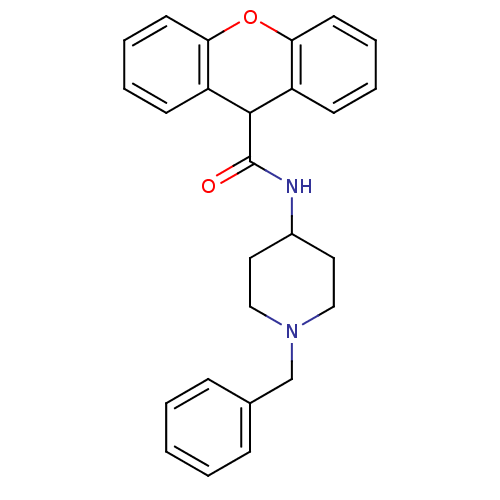
(9H-Xanthene-9-carboxylic acid (1-benzyl-piperidin-...)Show SMILES O=C(NC1CCN(Cc2ccccc2)CC1)C1c2ccccc2Oc2ccccc12 Show InChI InChI=1S/C26H26N2O2/c29-26(27-20-14-16-28(17-15-20)18-19-8-2-1-3-9-19)25-21-10-4-6-12-23(21)30-24-13-7-5-11-22(24)25/h1-13,20,25H,14-18H2,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113085
BindingDB Entry DOI: 10.7270/Q2BC43MB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data