

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 2B (Homo sapiens (Human)) | BDBM264089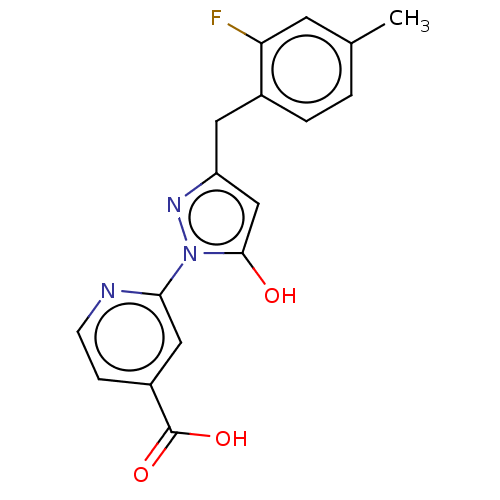 (2-[3-[(2-fluoro-4- methylphenyl)methyl]-5- hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of FBXL10 was determined in 384-well plate format under the following reaction conditions: 0.3 ... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2B (Homo sapiens (Human)) | BDBM264090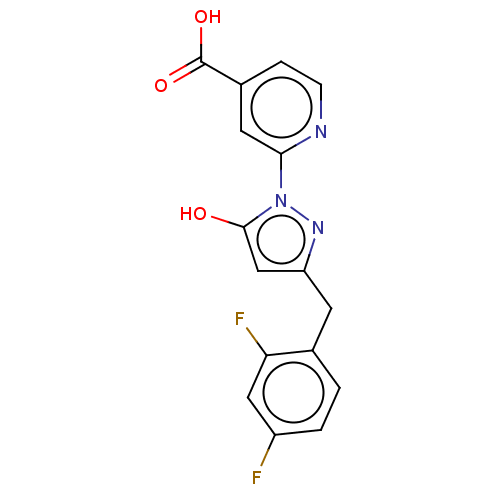 (2-[3-[(2,4-difluorophenyl)methyl]- 5-hydroxypyrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of FBXL10 was determined in 384-well plate format under the following reaction conditions: 0.3 ... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50158755 (CHEMBL3786579 | US10173996, Example 109 | US960496...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264025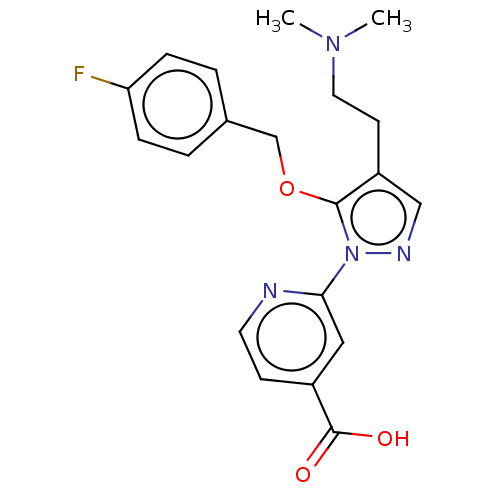 (2-(4-[2-(dimethylamino)ethyl]- 5-[(4- fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264025 (2-(4-[2-(dimethylamino)ethyl]- 5-[(4- fluorophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264030 (2-[5-[(4-chloro-2- ethoxyphenyl)methoxy]pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264030 (2-[5-[(4-chloro-2- ethoxyphenyl)methoxy]pyrazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264031 (2-[5-[[4-chloro-2-(2- methoxyethoxy)phenyl]methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264031 (2-[5-[[4-chloro-2-(2- methoxyethoxy)phenyl]methoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264033 (2-[5-[[4-fluoro-2-[(4- fluorophenyl)methoxy]phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264033 (2-[5-[[4-fluoro-2-[(4- fluorophenyl)methoxy]phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264038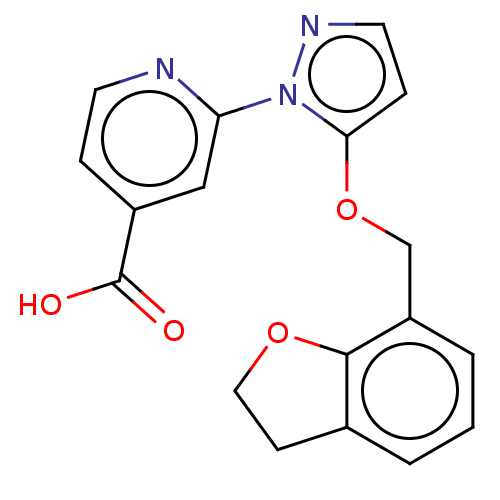 (2-[5-(2,3-dihydro-1-benzofuran-7- ylmethoxy)pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264038 (2-[5-(2,3-dihydro-1-benzofuran-7- ylmethoxy)pyrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264039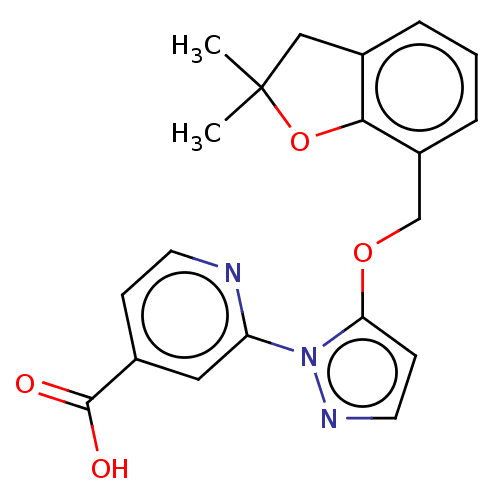 (2-[5-[(2,2-dimethyl-3H-1- benzofuran-7-yl)methoxy]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264040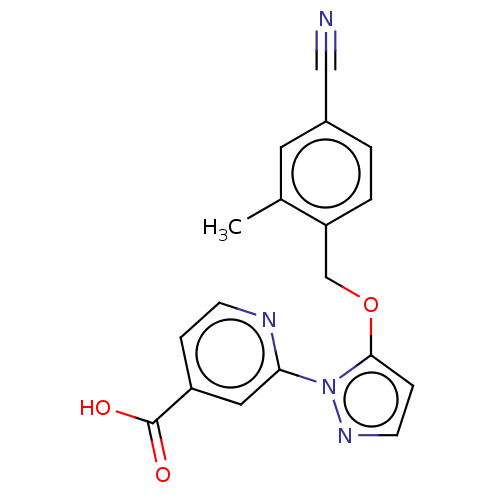 (2-[5-[(4-cyano-2- methylphenyl)methoxy]pyrazol-1- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264040 (2-[5-[(4-cyano-2- methylphenyl)methoxy]pyrazol-1- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264041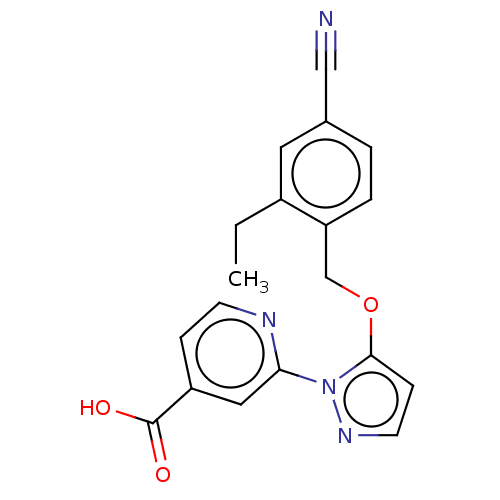 (2-[5-[(4-cyano-2- ethylphenyl)methoxy]pyrazol-1- y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264041 (2-[5-[(4-cyano-2- ethylphenyl)methoxy]pyrazol-1- y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264043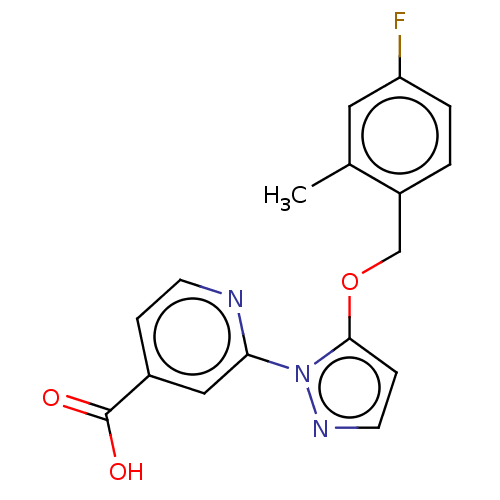 (2-[5-[(4-fluoro-2- methylphenyl)methoxy]pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264043 (2-[5-[(4-fluoro-2- methylphenyl)methoxy]pyrazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264044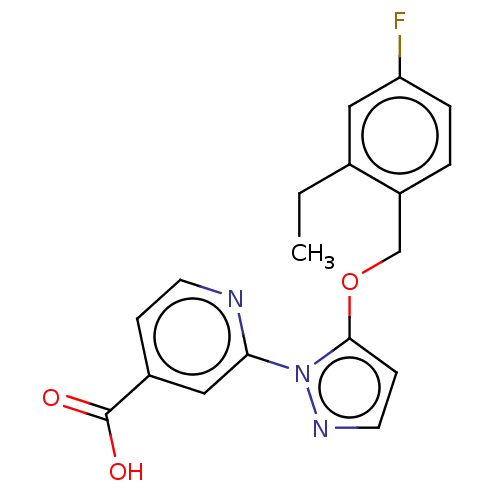 (2-[5-[(2-ethyl-4- fluorophenyl)methoxy]pyrazol-1- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264044 (2-[5-[(2-ethyl-4- fluorophenyl)methoxy]pyrazol-1- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264046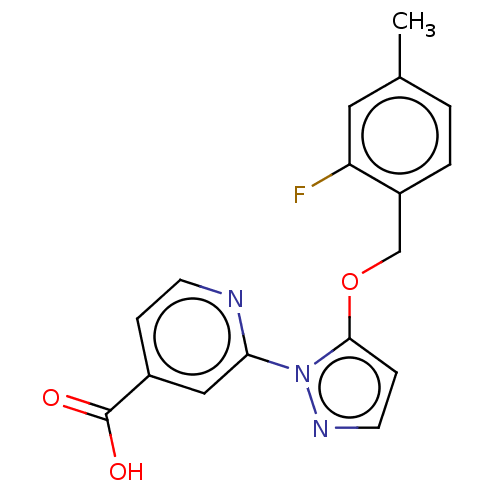 (2-[5-[(2-fluoro-4- methylphenyl)methoxy]pyrazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264046 (2-[5-[(2-fluoro-4- methylphenyl)methoxy]pyrazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264047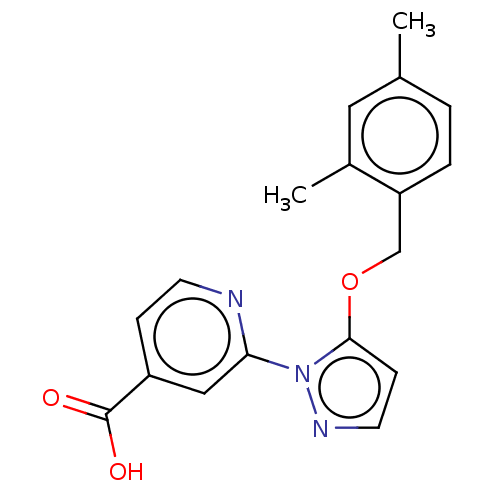 (2-[5-[(2,4- dimethylphenyl)methoxy]pyrazol- 1-yl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264047 (2-[5-[(2,4- dimethylphenyl)methoxy]pyrazol- 1-yl]p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264048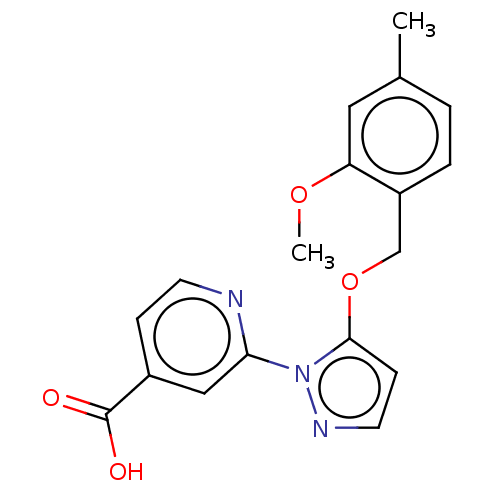 (2-[5-[(2-methoxy-4- methylphenyl)methoxy]pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264048 (2-[5-[(2-methoxy-4- methylphenyl)methoxy]pyrazol-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264049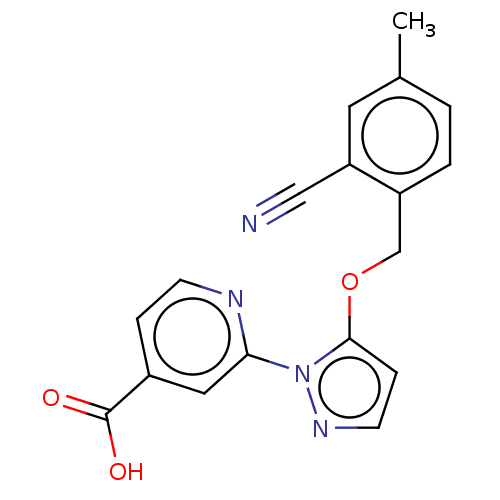 (2-[5-[(2-cyano-4- methylphenyl)methoxy]pyrazol-1- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264049 (2-[5-[(2-cyano-4- methylphenyl)methoxy]pyrazol-1- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264053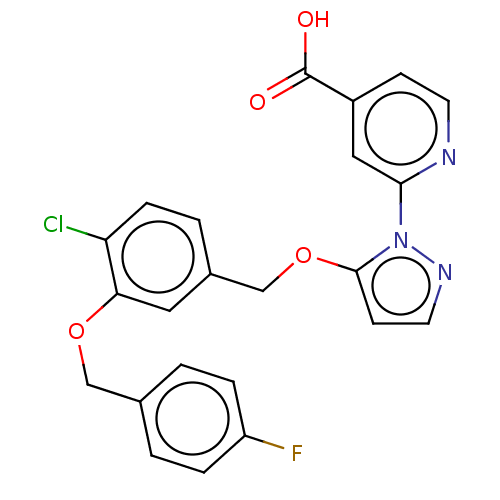 (2-[5-[[4-chloro-3-[(4- fluorophenyl)methoxy]phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264053 (2-[5-[[4-chloro-3-[(4- fluorophenyl)methoxy]phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264054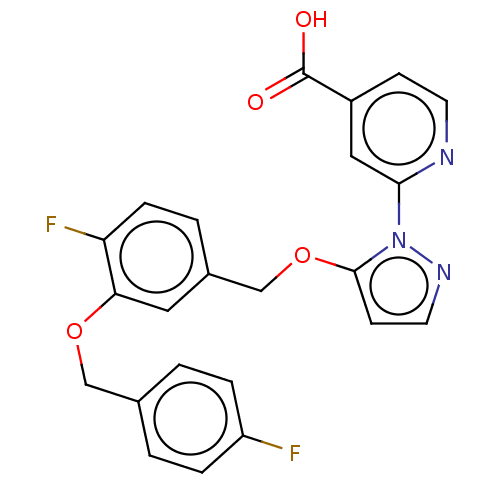 (2-[5-[[4-fluoro-3-[(4- fluorophenyl)methoxy]phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264054 (2-[5-[[4-fluoro-3-[(4- fluorophenyl)methoxy]phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264056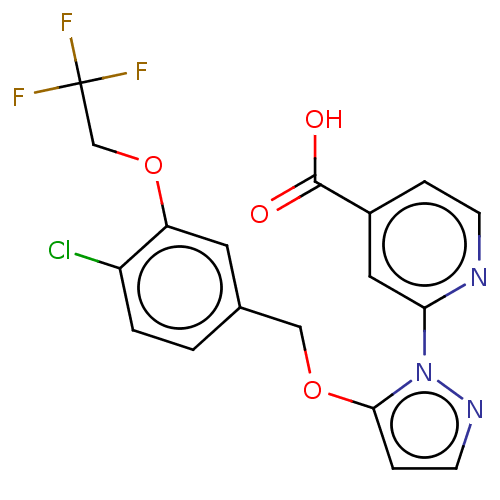 (2-[5-[[4-chloro-3-(2,2,2- trifluoroethoxy)phenyl]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264056 (2-[5-[[4-chloro-3-(2,2,2- trifluoroethoxy)phenyl]m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264058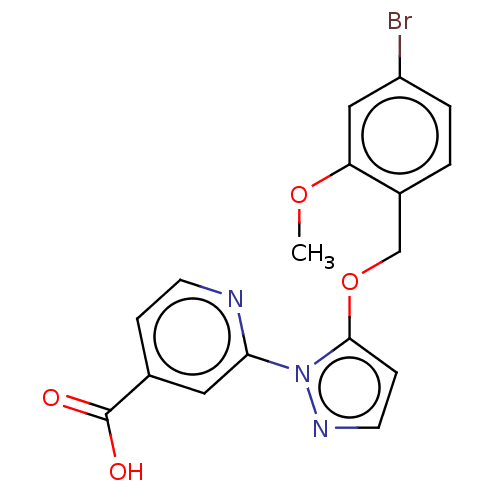 (2-[5-[(4-bromo-2- methoxyphenyl)methoxy]pyrazol- 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264058 (2-[5-[(4-bromo-2- methoxyphenyl)methoxy]pyrazol- 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264059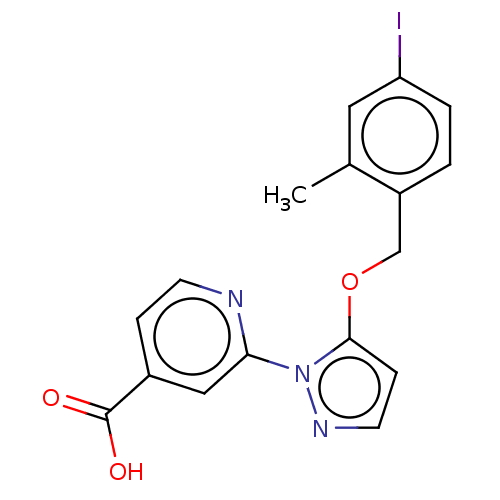 (2-[5-[(4-iodo-2- methylphenyl)methoxy]pyrazol- 1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264059 (2-[5-[(4-iodo-2- methylphenyl)methoxy]pyrazol- 1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264060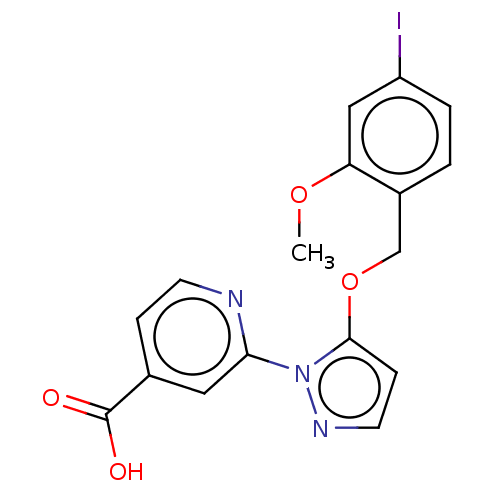 (2-[5-[(4-iodo-2- methoxyphenyl)methoxy]pyrazol- 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264060 (2-[5-[(4-iodo-2- methoxyphenyl)methoxy]pyrazol- 1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264064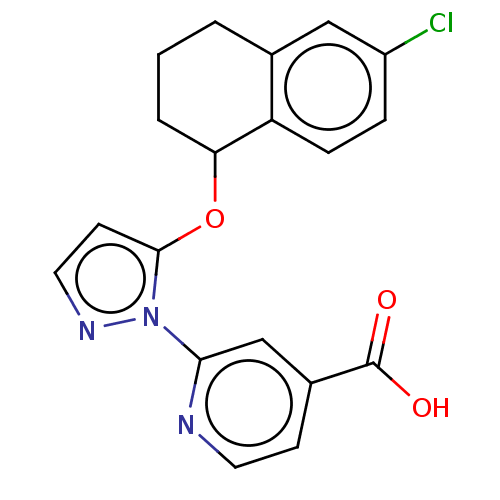 (2-[5-[(6-chloro-1,2,3,4- tetrahydronaphthalen-1- y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264066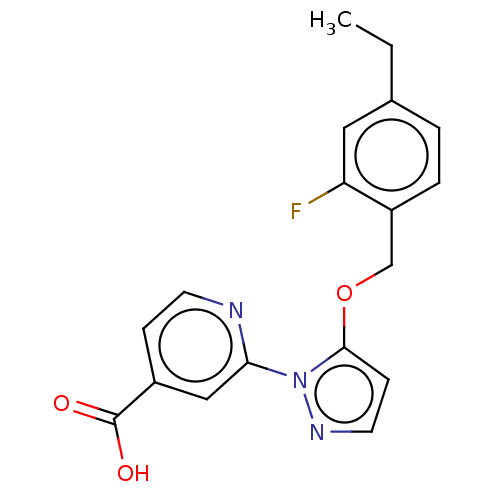 (2-[5-[(4-ethyl-2- fluorophenyl)methoxy]pyrazol-1- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264070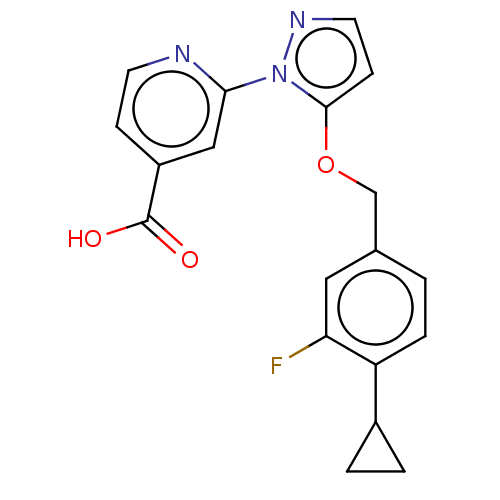 (2-[5-[(4-cyclopropyl-3- fluorophenyl)methoxy]pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM264071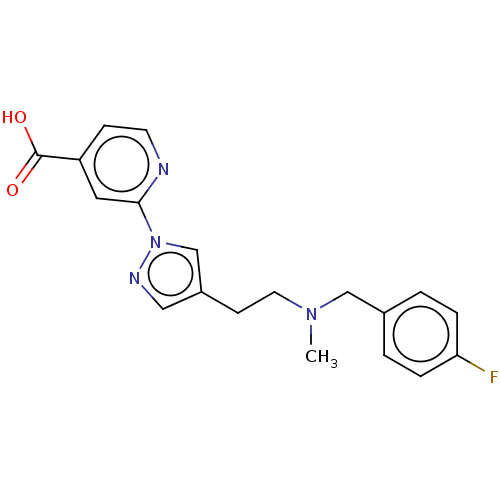 (2-[4-(2-[(4-fluorophenyl)methyl- methylamino]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM264071 (2-[4-(2-[(4-fluorophenyl)methyl- methylamino]ethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM264071 (2-[4-(2-[(4-fluorophenyl)methyl- methylamino]ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of JMJD2C was determined in 384-well plate format under the following reaction conditions: 0.3 ... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM375615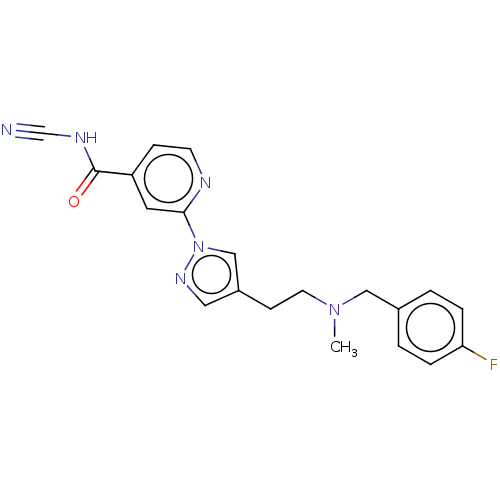 (N-cyano-2-[4-[2-[(4- fluorophenyl)methyl- methylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The enzymatic assay of Jarid1A activity is based upon Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The ability of test c... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM375615 (N-cyano-2-[4-[2-[(4- fluorophenyl)methyl- methylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 511 total ) | Next | Last >> |