

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50130915 (CHEBI:3122 | CHEMBL1231178) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50084040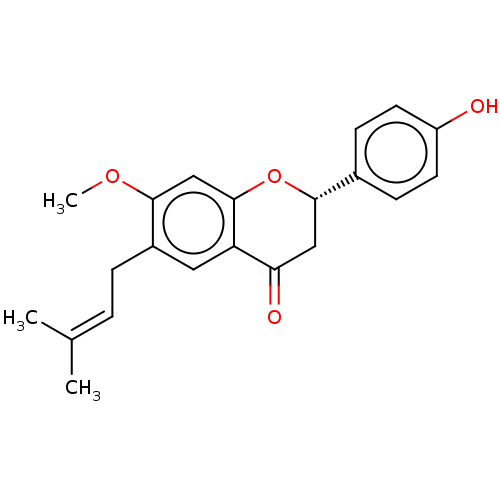 (Bavachinin A) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154555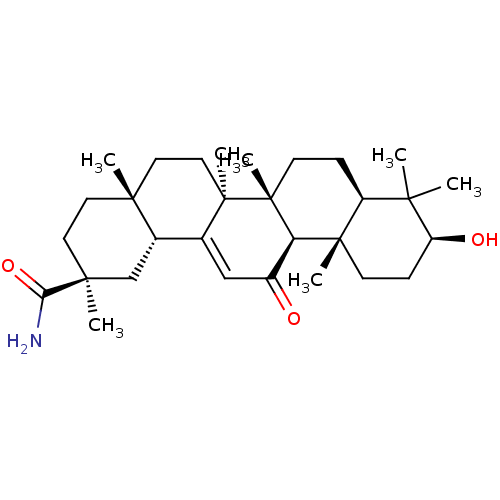 (CHEMBL3775916) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154554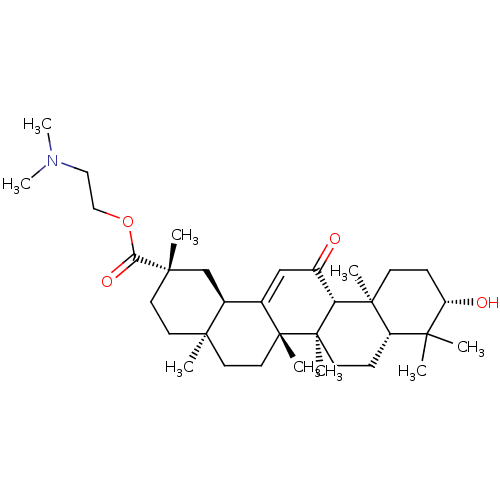 (CHEMBL3775391) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50346605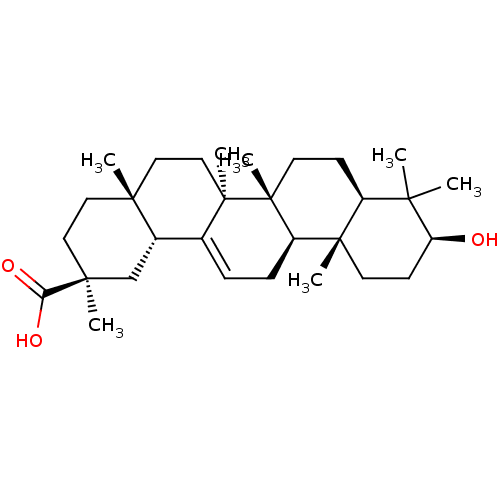 (CHEMBL487933) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154556 (CHEMBL1271200) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154558 (CHEMBL3775320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154561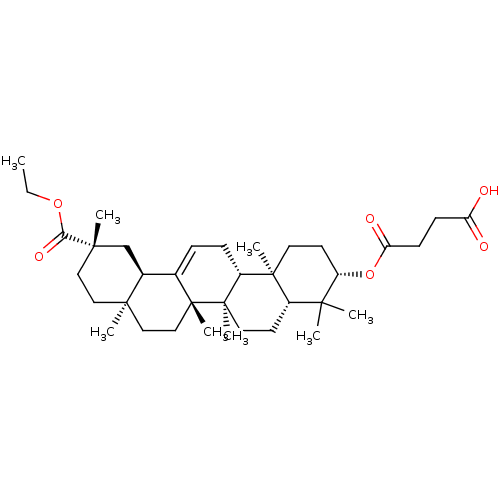 (CHEMBL3774603) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50058817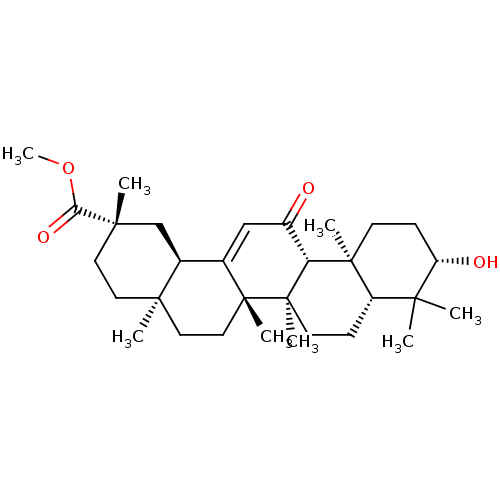 (CHEMBL1271483) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154563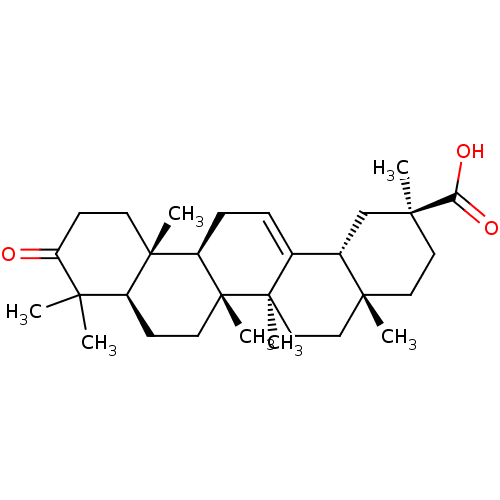 (CHEMBL3775913) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154560 (CHEMBL3775118) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154562 (CHEMBL1271536) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50188385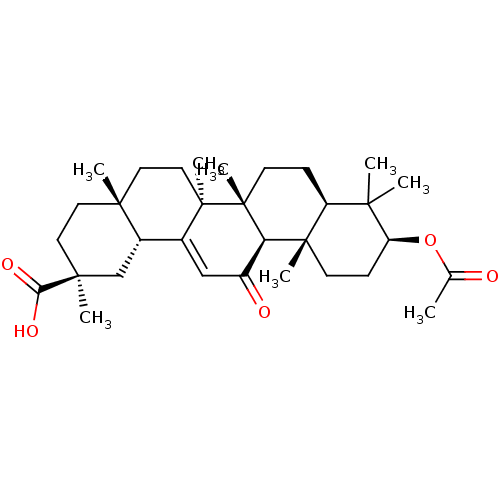 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154557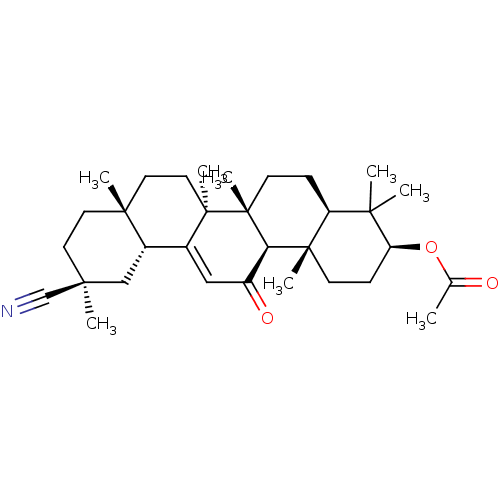 (CHEMBL3775481) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50154559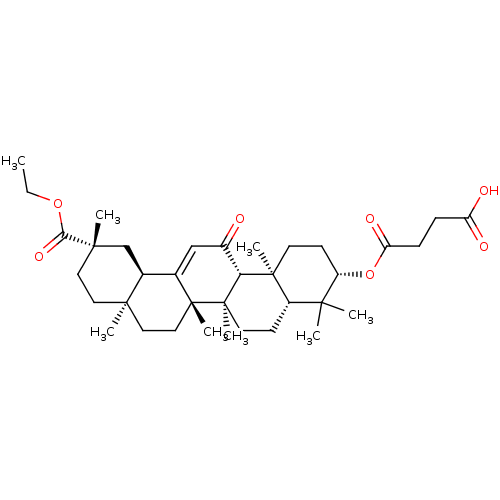 (CHEMBL2151605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50329190 ((18beta,20beta)-3,11-Dioxo-olean-12-en-29-oic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Inhibition of CE1 in human liver microsomes using 2-(2-Benzoyl-3-methoxyphenyl) benzothiazole as substrate preincubated for 10 mins followed by subst... | Eur J Med Chem 112: 280-8 (2016) Article DOI: 10.1016/j.ejmech.2016.02.020 BindingDB Entry DOI: 10.7270/Q2TQ63D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||