

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50160879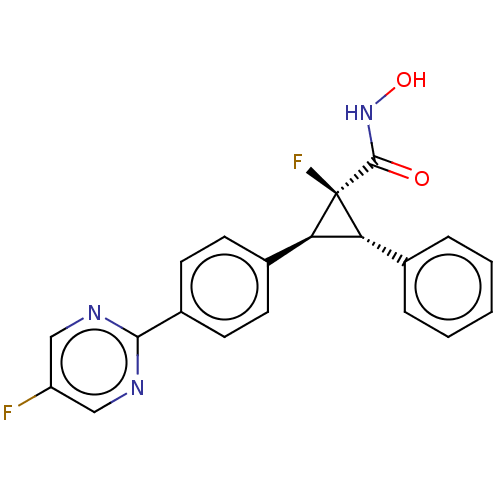 (CHEMBL3793392 | US9505736, (1S,2S,3S)-1-Fluoro-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257503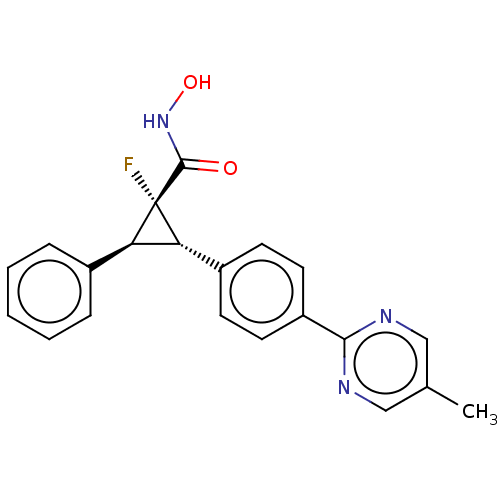 (US9505736, (1S,2S,3S)-1-Fluoro-N-hydroxy-2-(4-(5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257508 (US9505736, (1S,2S,3S)-2-(4-(2-Cyclopropyloxazol- 5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50161831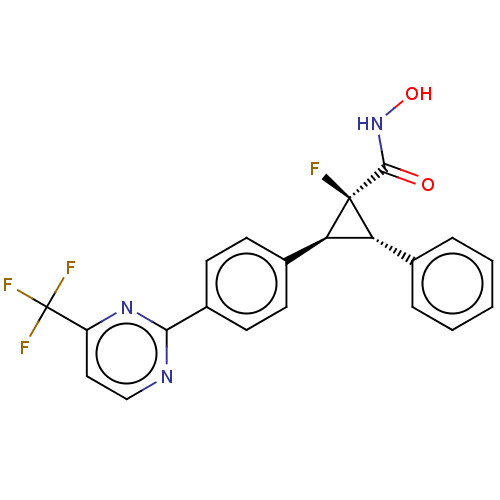 (CHEMBL3793932 | US9505736, (1S,2S,3S)-1-Fluoro-N-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50160874 (CHEMBL3794485 | US9505736, (1S,2S,3S)-2-(4-(5- (Di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257501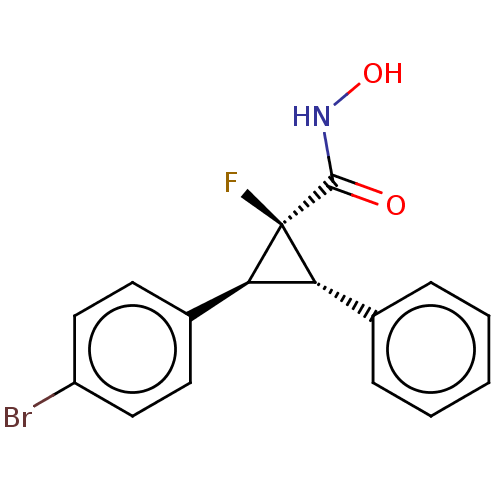 (US9505736, (1S,2S,3S)-2-(4-Bromophenyl)-1- fluoro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257509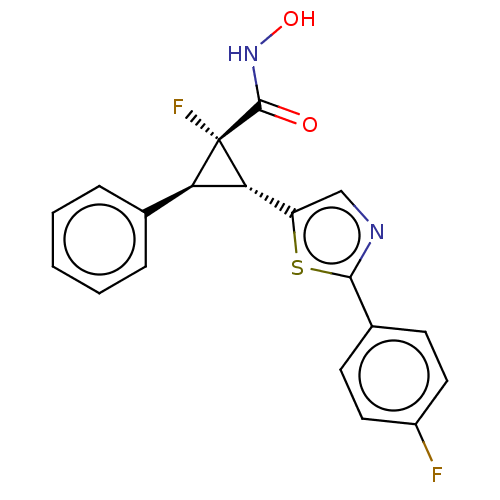 (US9505736, (1S,2R,3S)-1-Fluoro-2-(2-(4- fluorophen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50155803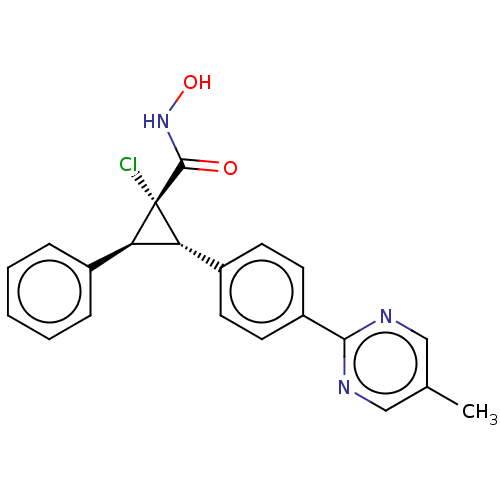 (CHEMBL3793455 | US9505736, (1S,2S,3S)-1-Chloro-N-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257511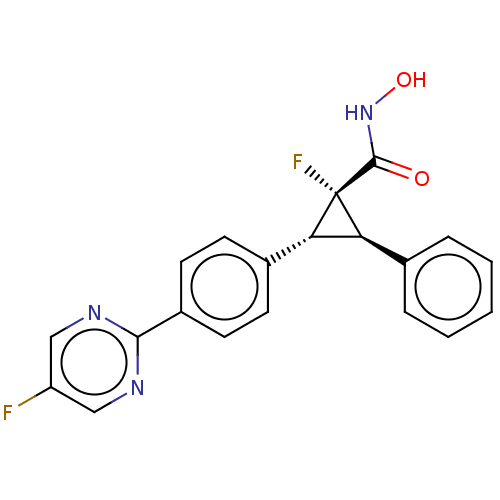 (US9505736, (1R,2R,3R)-1-Fluoro-2-(4-(5- fluoropyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257505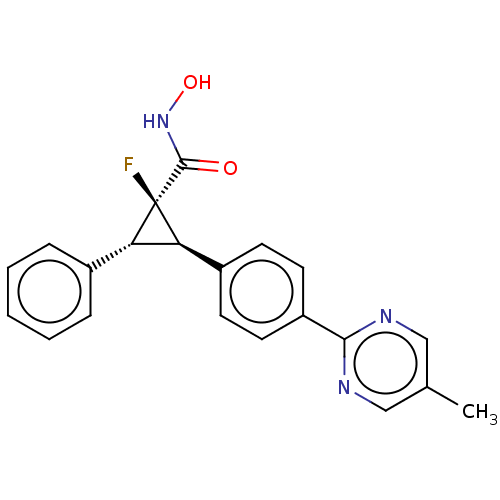 (US9505736, (1R,2R,3R)-1-Fluoro-N-hydroxy-2-(4-(5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257504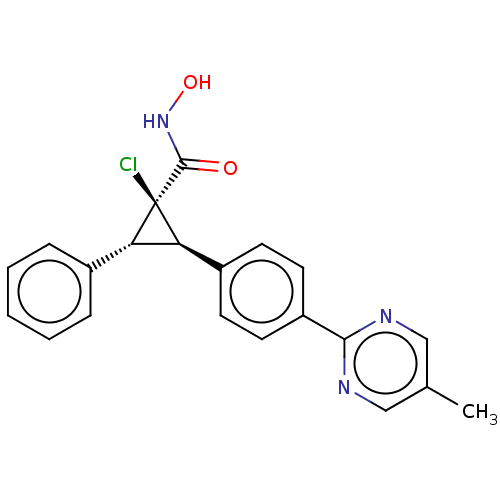 (US9505736, (1R,2R,3R)-1-Chloro-N-hydroxy-2-(4-(5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257507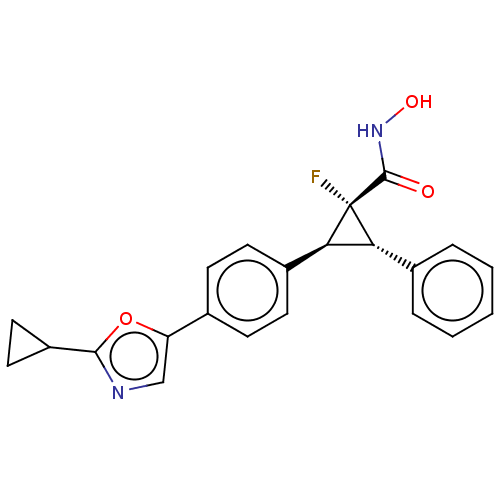 (US9505736, (1R,2S,3S)-2-(4-(2-Cyclopropyloxazol- 5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257502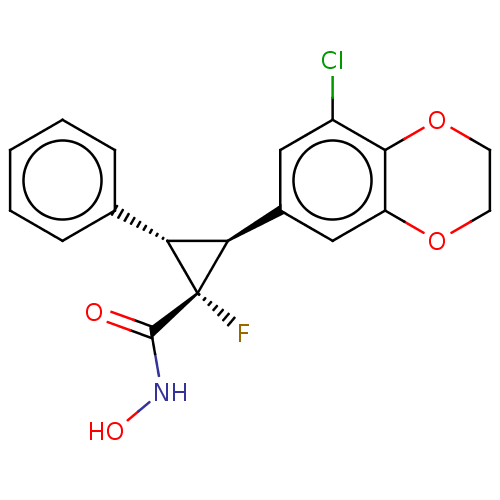 (US9505736, (1R,2S,3S)-2-(8-Chloro-2,3- dihydrobenz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.47E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257510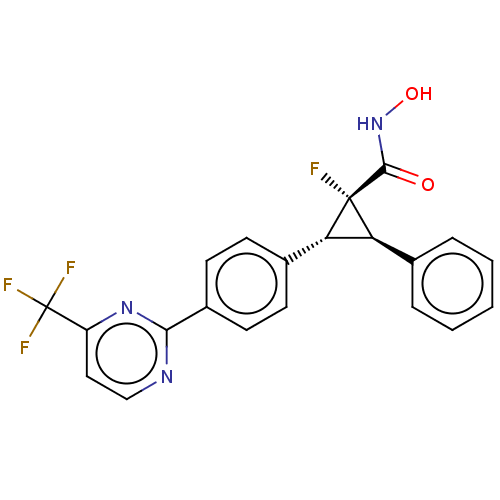 (US9505736, (1R,2R,3R)-1-Fluoro-N-hydroxy-2-phenyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.87E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257506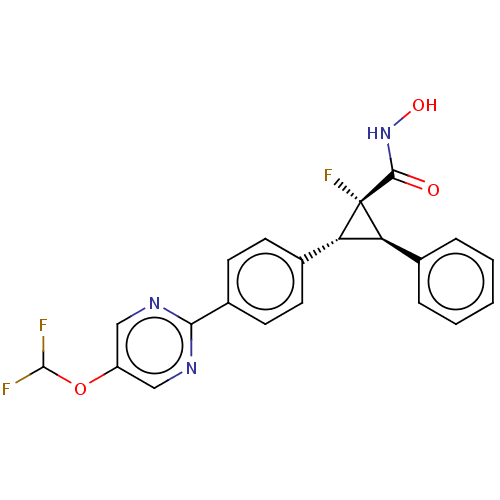 (US9505736, (1R,2R,3R)-2-(4-(5- (Difluoromethoxy)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM257512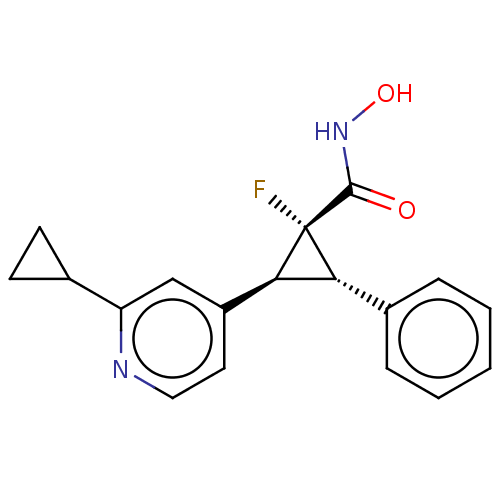 (US9505736, (1R,2S,3S)-2-(2-Cyclopropylpyridin-4- y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||