

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384894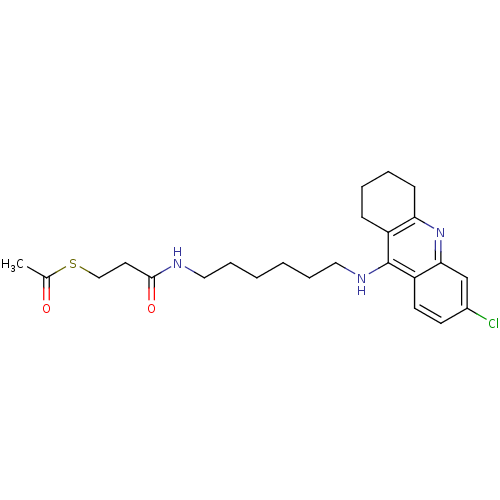 (CHEMBL2036262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384893 (CHEMBL2036261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384892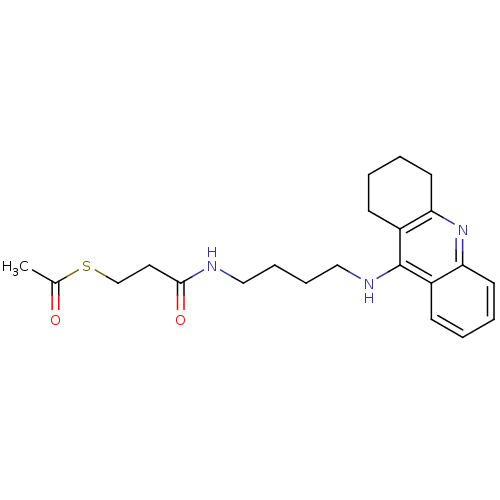 (CHEMBL2036260) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384890 (CHEMBL2036263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384895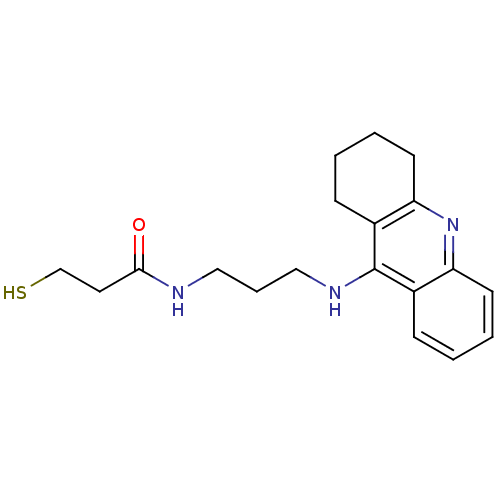 (CHEMBL2036259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384891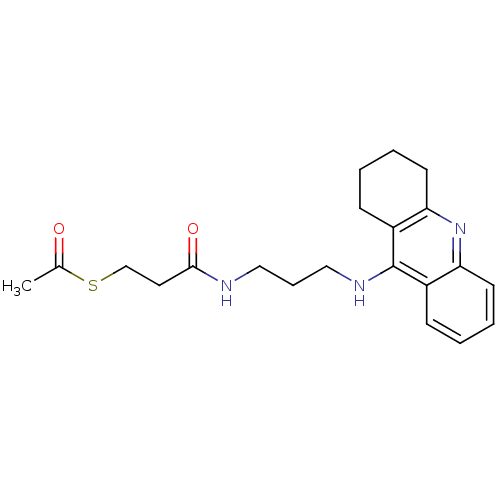 (CHEMBL2036258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||