

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199337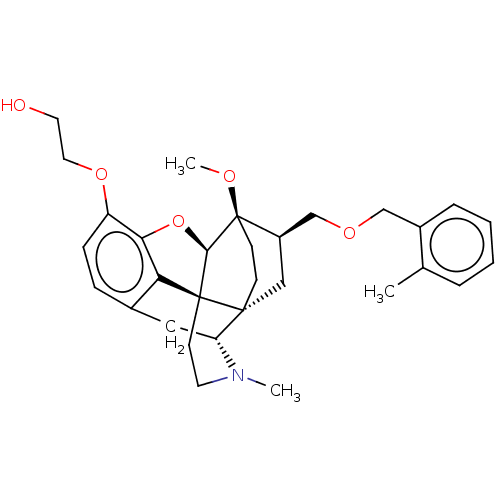 (US9221831, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.94 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199338 (US9221831, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 40.4 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199339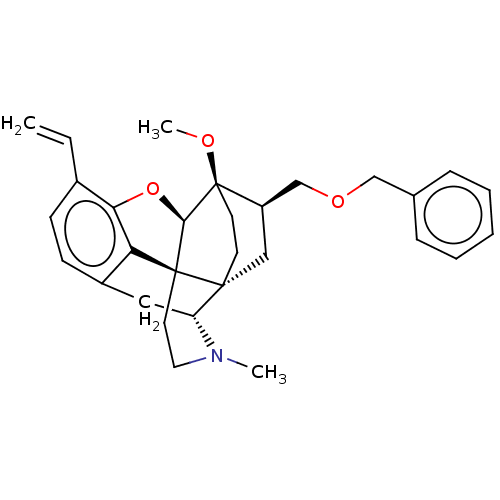 (US9221831, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.82 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199340 (US9221831, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 22.8 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199341 (US9221831, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.46E+3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199342 (US9221831, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 577 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199343 (US9221831, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 63.8 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199344 (US9221831, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 129 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199345 (US9221831, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 890 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM4203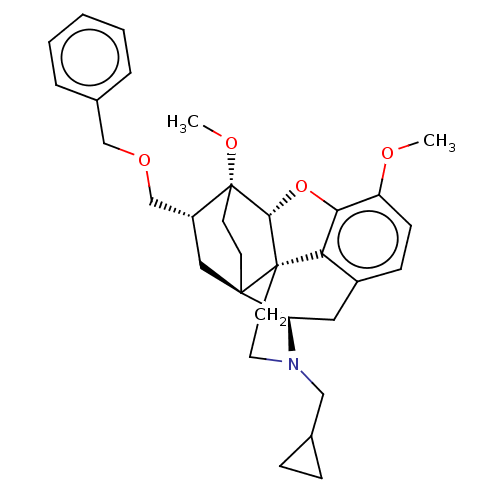 (US8530494, 6 | US9221831, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 4.27 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199348 (US9221831, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.03E+4 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199349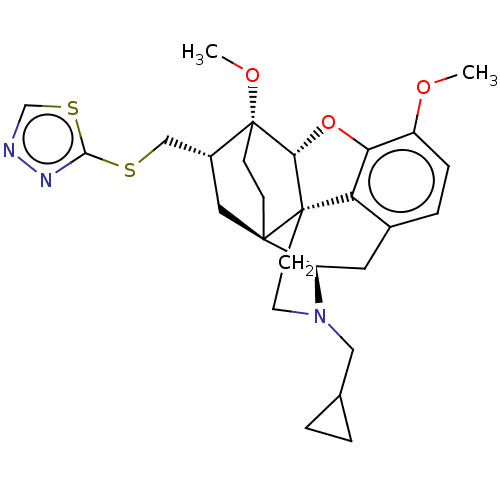 (US9221831, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 192 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199350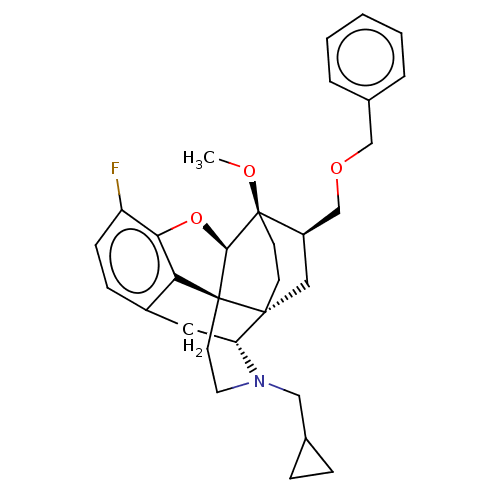 (US9221831, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199351 (US9221831, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 70.7 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199352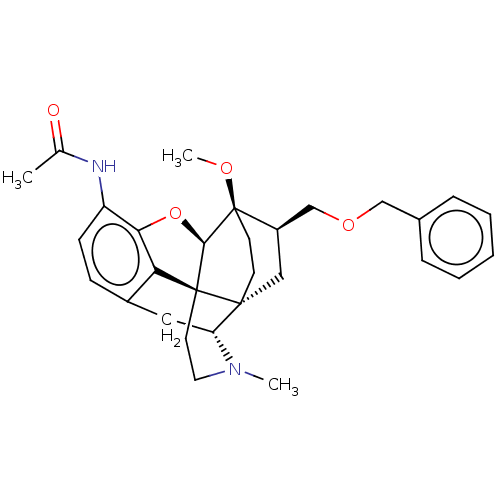 (US9221831, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 951 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199353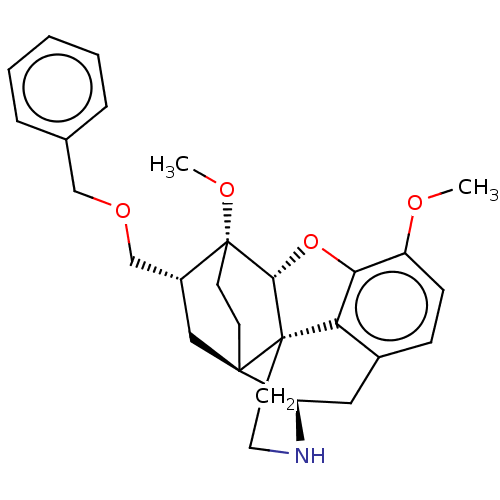 (US9221831, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 22.5 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199354 (US9221831, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9.46 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199355 (US9221831, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 86.5 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199356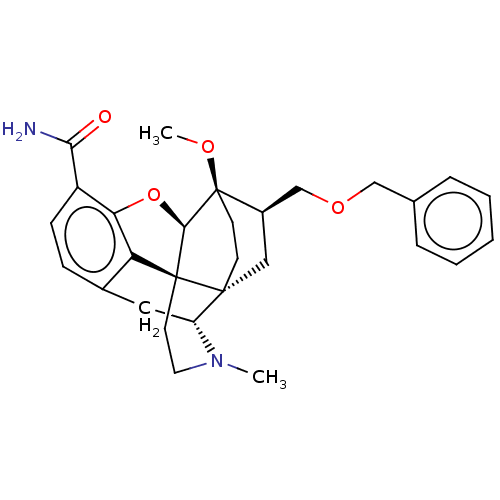 (US9221831, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 13.3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199357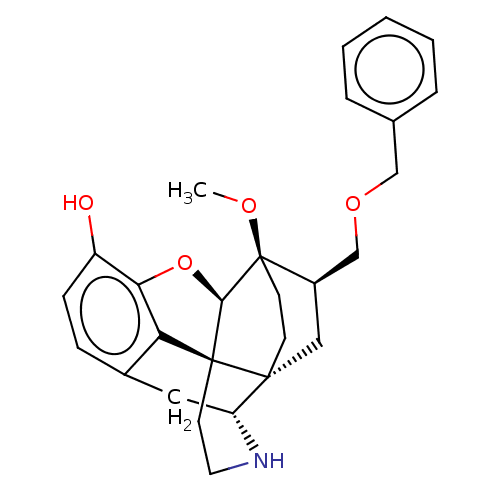 (US9221831, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.840 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199358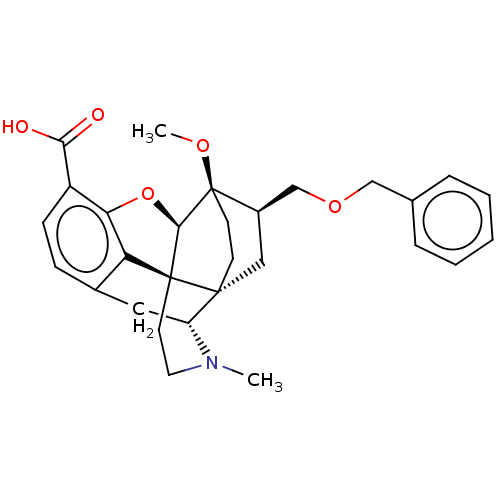 (US9221831, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 156 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199359 (US9221831, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 196 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199360 (US9221831, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.62 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199361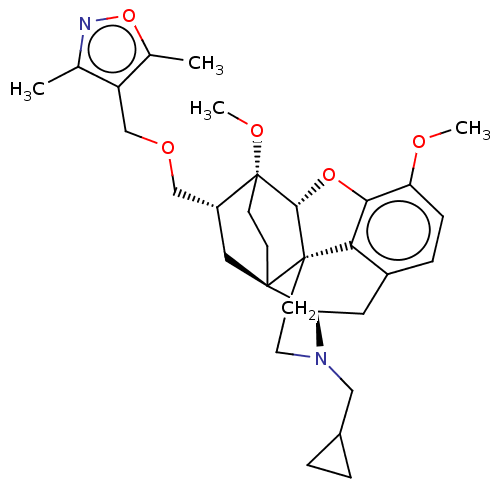 (US9221831, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 21.1 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199362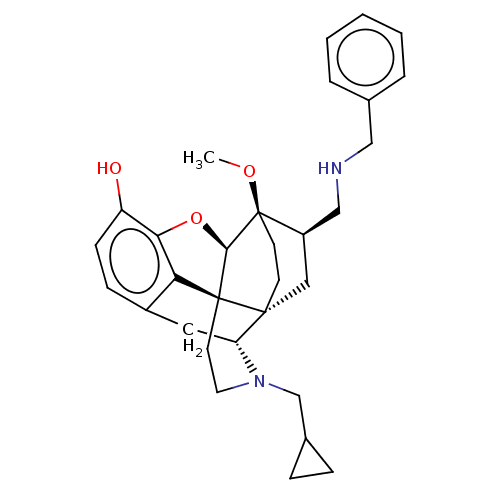 (US9221831, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 108 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199363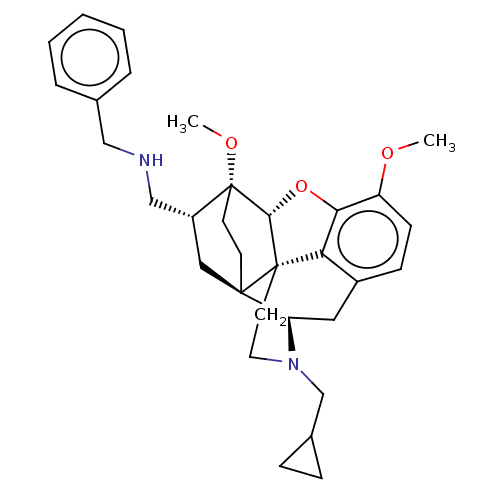 (US9221831, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.42 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199364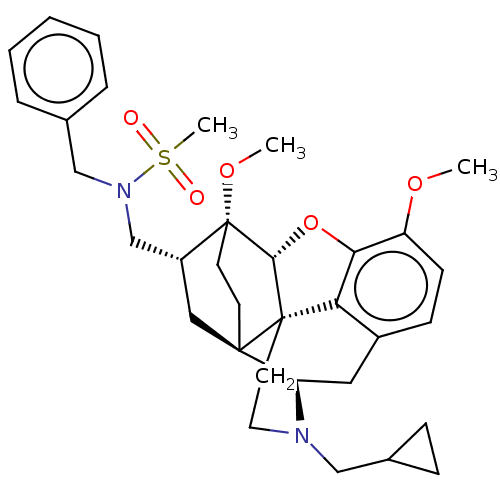 (US9221831, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 80.5 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199365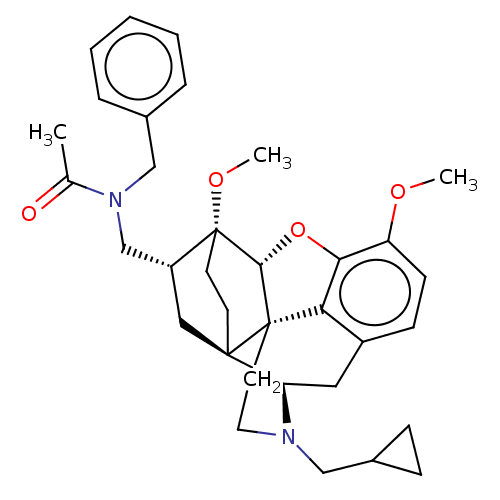 (US9221831, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.13 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199366 (US9221831, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.29 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199367 (US9221831, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9.08 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199368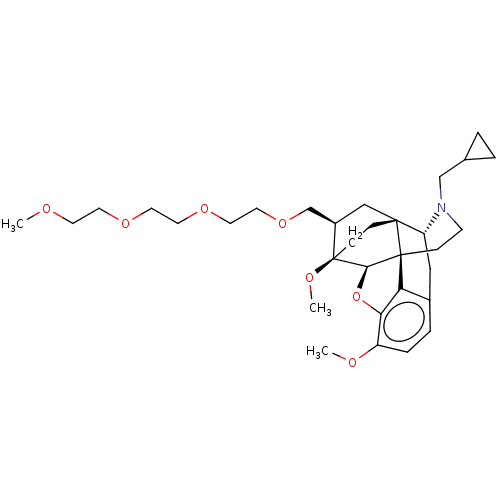 (US9221831, 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 664 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199369 (US9221831, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 817 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199370 (US9221831, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11.4 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199371 (US9221831, 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 42.2 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199372 (US9221831, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.64E+3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199374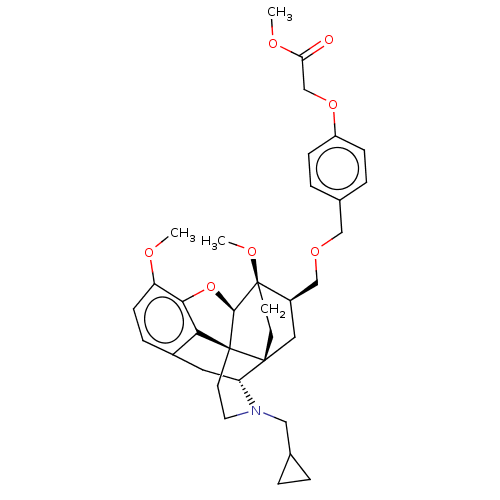 (US9221831, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 95.1 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199375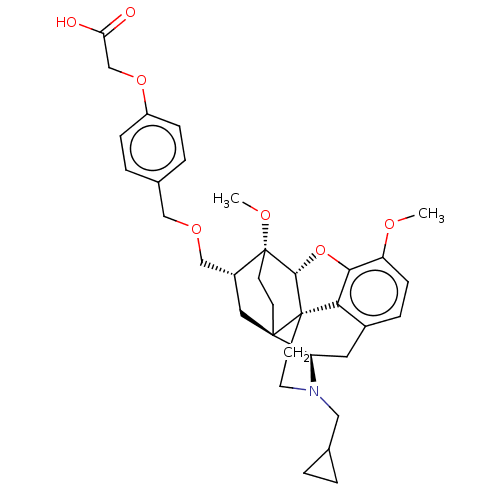 (US9221831, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 202 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199376 (US9221831, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 51.9 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199377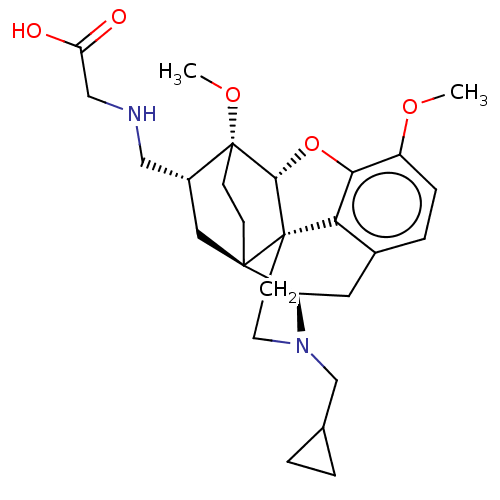 (US9221831, 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 964 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199379 (US9221831, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199380 (US9221831, 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.44E+3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199381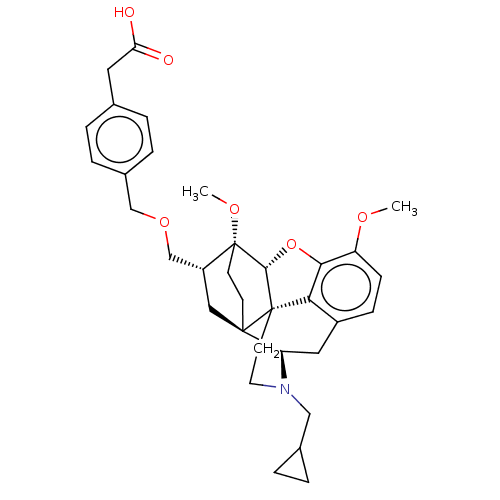 (US9221831, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 901 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199382 (US9221831, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 462 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199383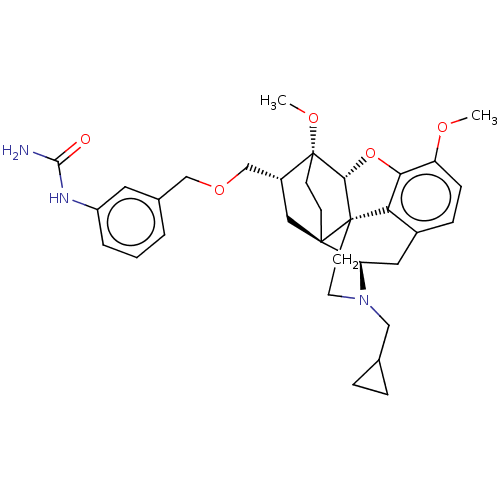 (US9221831, 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 53.5 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199384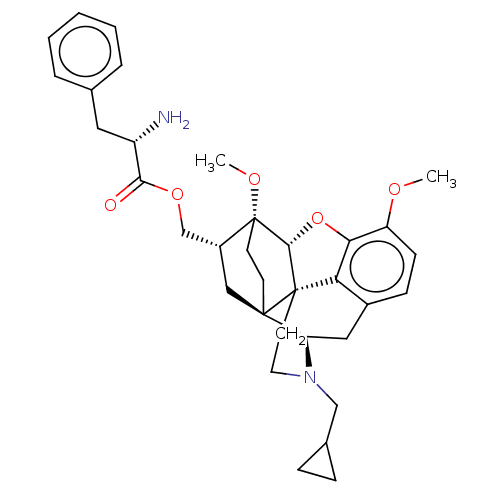 (US9221831, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 82.3 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199385 (US9221831, 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 129 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199386 (US9221831, 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 14.6 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199390 (US9221831, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 645 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199391 (US9221831, 56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 513 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM199392 (US9221831, 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 418 | n/a | n/a | 7.4 | 25 |
Purdue Pharma, L.P. US Patent | Assay Description Functional [35S]GTPgammaS binding assays were conducted as follows. κ opioid receptor membrane solution was prepared by sequentially adding final ... | US Patent US9221831 (2015) BindingDB Entry DOI: 10.7270/Q2T72G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |