 Found 86 hits of kd data for polymerid = 2556
Found 86 hits of kd data for polymerid = 2556 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595335

(CHEMBL5181059)Show SMILES COc1ccc(Cn2ccc3ccc(cc23)C(=O)NO)cc1C(=O)NCCCCNc1ccc(c2nonc12)S(=O)(=O)N(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116821
BindingDB Entry DOI: 10.7270/Q2CJ8JGG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595334

(CHEMBL5202271)Show SMILES COc1ccc(Cn2ccc3ccc(cc23)C(=O)NO)cc1C(=O)NCCCNc1ccc(c2nonc12)S(=O)(=O)N(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116821
BindingDB Entry DOI: 10.7270/Q2CJ8JGG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50595333

(CHEMBL5197578)Show SMILES COc1ccc(Cn2ccc3ccc(cc23)C(=O)NO)cc1C(=O)NCCNc1ccc(c2nonc12)S(=O)(=O)N(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116821
BindingDB Entry DOI: 10.7270/Q2CJ8JGG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50377687
(CHEMBL255686)Show SMILES ONC(=O)CCCCCCNC(=O)c1ccc(NCc2cn(CCCNC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccccc4oc4cc(=O)ccc34)nn2)cc1 |(33.95,-8.48,;32.41,-8.48,;31.64,-7.15,;32.4,-5.81,;30.1,-7.15,;29.32,-5.82,;27.78,-5.82,;27.01,-4.48,;25.47,-4.48,;24.7,-3.15,;23.16,-3.15,;22.39,-1.81,;23.16,-.48,;20.85,-1.81,;20.08,-3.15,;18.54,-3.15,;17.79,-1.81,;16.25,-1.81,;15.48,-.47,;13.94,-.47,;12.9,.67,;11.5,.03,;10.17,.8,;8.83,.02,;7.5,.79,;6.17,.02,;4.83,.78,;4.83,2.32,;3.5,.01,;3.5,-1.53,;2.18,-2.3,;2.17,-3.85,;3.51,-4.62,;4.84,-3.85,;4.84,-2.3,;6.18,-4.61,;6.18,-6.15,;7.51,-3.84,;3.48,-7.08,;4.8,-7.85,;6.13,-7.07,;7.46,-7.83,;7.47,-9.38,;6.13,-10.16,;4.79,-9.38,;3.47,-10.15,;2.14,-9.37,;.82,-10.14,;-.51,-9.37,;-1.84,-10.14,;-.51,-7.84,;.82,-7.08,;2.14,-7.84,;11.66,-1.49,;13.17,-1.81,;18.54,-.48,;20.08,-.48,)| Show InChI InChI=1S/C41H42N8O7S/c50-30-16-18-33-36(23-30)56-35-9-5-4-8-32(35)38(33)31-17-15-28(22-34(31)40(53)54)45-41(57)43-20-7-21-49-25-29(46-48-49)24-44-27-13-11-26(12-14-27)39(52)42-19-6-2-1-3-10-37(51)47-55/h4-5,8-9,11-18,22-23,25,44,55H,1-3,6-7,10,19-21,24H2,(H,42,52)(H,47,51)(H,53,54)(H2,43,45,57) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a |
The Broad Institute of Harvard and MIT
Curated by ChEMBL
| Assay Description
Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3... |
Bioorg Med Chem Lett 18: 2809-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.007
BindingDB Entry DOI: 10.7270/Q2ST7QR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50567882
(CHEMBL4871247)Show SMILES Cc1ccc(s1)-c1nc(CNc2ccc(cc2)C(=O)NO)n(n1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human HDAC6 by BLI assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113392
BindingDB Entry DOI: 10.7270/Q2611436 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361256
(CHEMBL1934898)Show SMILES CCc1cccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)NO)c1 Show InChI InChI=1S/C16H12F12N2O3/c1-2-7-4-3-5-8(6-7)29-9(31)11(17,18)13(21,22)15(25,26)16(27,28)14(23,24)12(19,20)10(32)30-33/h3-6,33H,2H2,1H3,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50455887
(CHEMBL4204982)Show InChI InChI=1S/C12H12N2O3/c1-14-10(6-7-11(15)16)13-9-5-3-2-4-8(9)12(14)17/h2-5H,6-7H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated HDAC6 ZnF-UBD (unknown origin) by surface plasmon resonance method |
J Med Chem 61: 4517-4527 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00258
BindingDB Entry DOI: 10.7270/Q2T72M2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361253

(CHEMBL1934895)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(I)cc1 Show InChI InChI=1S/C14H7F12IN2O3/c15-9(16,7(30)28-6-3-1-5(27)2-4-6)11(19,20)13(23,24)14(25,26)12(21,22)10(17,18)8(31)29-32/h1-4,32H,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361259
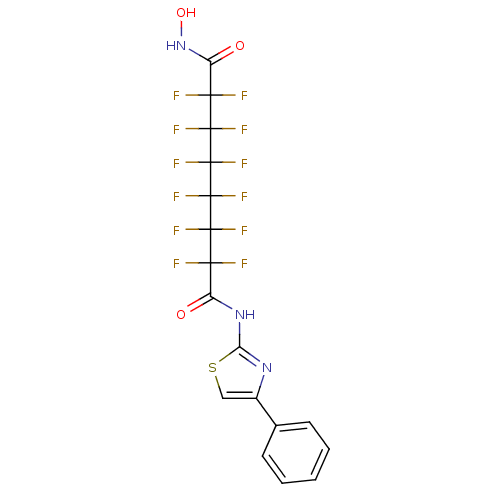
(CHEMBL1934901)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C17H9F12N3O3S/c18-12(19,9(33)31-11-30-8(6-36-11)7-4-2-1-3-5-7)14(22,23)16(26,27)17(28,29)15(24,25)13(20,21)10(34)32-35/h1-6,35H,(H,32,34)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361267
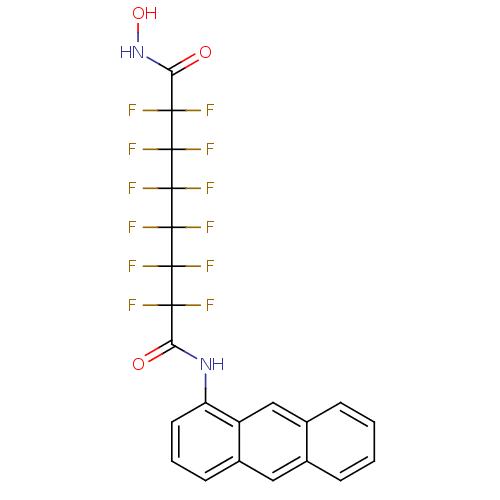
(CHEMBL1934909)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1cccc2cc3ccccc3cc12 Show InChI InChI=1S/C22H12F12N2O3/c23-17(24,19(27,28)21(31,32)22(33,34)20(29,30)18(25,26)16(38)36-39)15(37)35-14-7-3-6-12-8-10-4-1-2-5-11(10)9-13(12)14/h1-9,39H,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361263
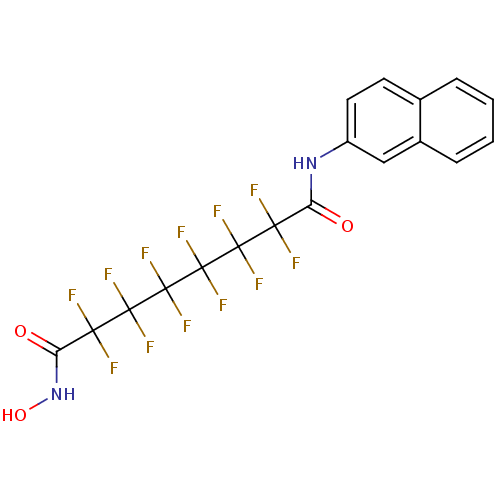
(CHEMBL1934905)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc2ccccc2c1 Show InChI InChI=1S/C18H10F12N2O3/c19-13(20,11(33)31-10-6-5-8-3-1-2-4-9(8)7-10)15(23,24)17(27,28)18(29,30)16(25,26)14(21,22)12(34)32-35/h1-7,35H,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411273

(CHEMBL5273203)Show SMILES Cc1cc(C)c2cccc(OCc3c(Cl)ccc(c3Cl)S(=O)(=O)NC3(CCCC3)C(=O)N3CCN(CC3)C(=O)C[N+](C)(C)C)c2n1 Show InChI InChI=1S/C33H42Cl2N5O5S/c1-22-19-23(2)36-31-24(22)9-8-10-27(31)45-21-25-26(34)11-12-28(30(25)35)46(43,44)37-33(13-6-7-14-33)32(42)39-17-15-38(16-18-39)29(41)20-40(3,4)5/h8-12,19,37H,6-7,13-18,20-21H2,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Human immunodeficiency virus 1 reverse transcriptase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50455900
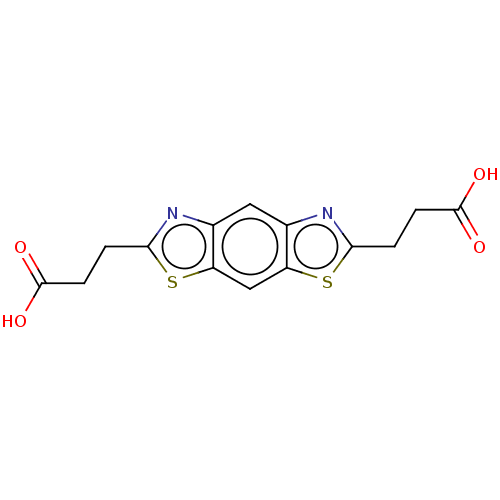
(CHEMBL4215676)Show InChI InChI=1S/C14H12N2O4S2/c17-13(18)3-1-11-15-7-5-8-10(6-9(7)21-11)22-12(16-8)2-4-14(19)20/h5-6H,1-4H2,(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated HDAC6 ZnF-UBD (unknown origin) by surface plasmon resonance method |
J Med Chem 61: 4517-4527 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00258
BindingDB Entry DOI: 10.7270/Q2T72M2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361257
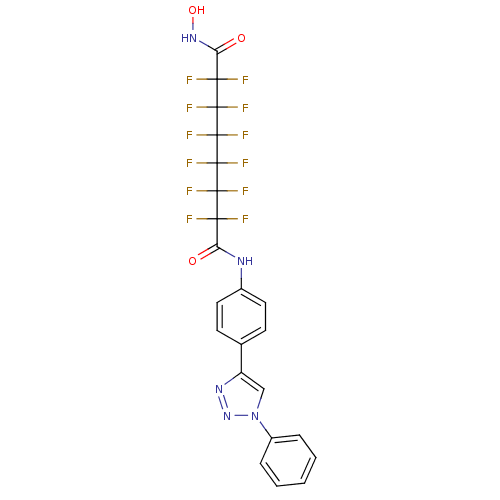
(CHEMBL1934899)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(cc1)-c1cn(nn1)-c1ccccc1 Show InChI InChI=1S/C22H13F12N5O3/c23-17(24,19(27,28)21(31,32)22(33,34)20(29,30)18(25,26)16(41)37-42)15(40)35-12-8-6-11(7-9-12)14-10-39(38-36-14)13-4-2-1-3-5-13/h1-10,42H,(H,35,40)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361264
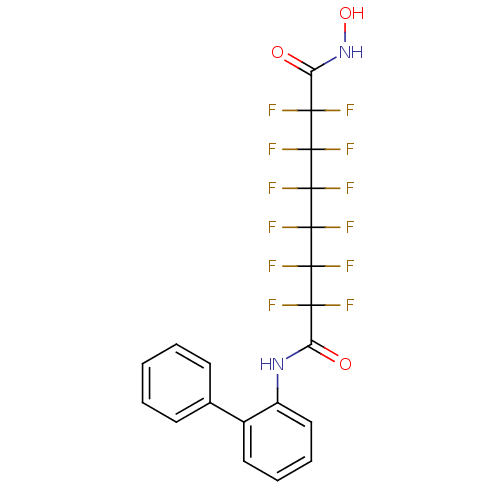
(CHEMBL1934906)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccccc1-c1ccccc1 Show InChI InChI=1S/C20H12F12N2O3/c21-15(22,13(35)33-12-9-5-4-8-11(12)10-6-2-1-3-7-10)17(25,26)19(29,30)20(31,32)18(27,28)16(23,24)14(36)34-37/h1-9,37H,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361251
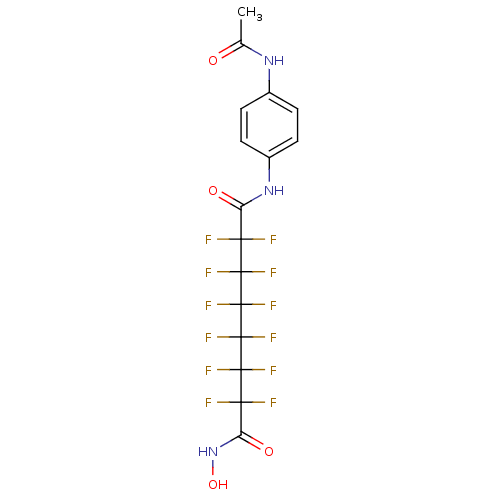
(CHEMBL1934893)Show SMILES CC(=O)Nc1ccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)NO)cc1 Show InChI InChI=1S/C16H11F12N3O4/c1-6(32)29-7-2-4-8(5-3-7)30-9(33)11(17,18)13(21,22)15(25,26)16(27,28)14(23,24)12(19,20)10(34)31-35/h2-5,35H,1H3,(H,29,32)(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361266
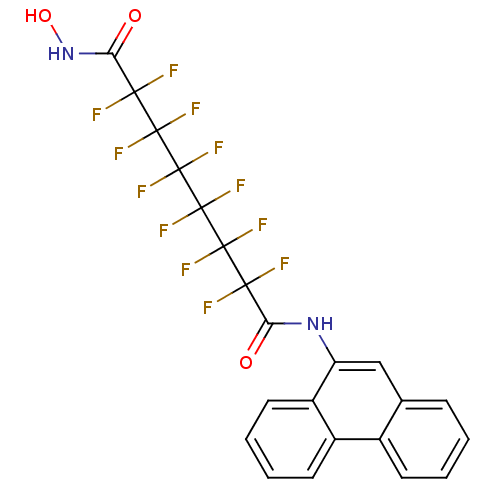
(CHEMBL1934908)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1cc2ccccc2c2ccccc12 Show InChI InChI=1S/C22H12F12N2O3/c23-17(24,19(27,28)21(31,32)22(33,34)20(29,30)18(25,26)16(38)36-39)15(37)35-14-9-10-5-1-2-6-11(10)12-7-3-4-8-13(12)14/h1-9,39H,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361248
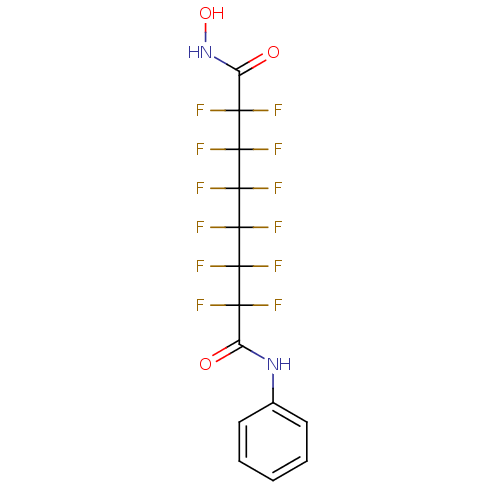
(CHEMBL1934890)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccccc1 Show InChI InChI=1S/C14H8F12N2O3/c15-9(16,7(29)27-6-4-2-1-3-5-6)11(19,20)13(23,24)14(25,26)12(21,22)10(17,18)8(30)28-31/h1-5,31H,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411309
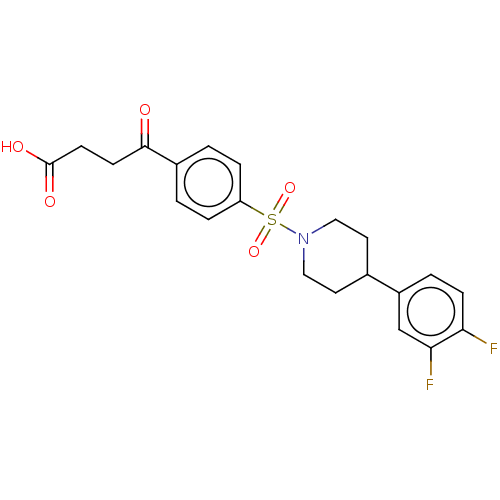
(CHEMBL5276559)Show SMILES C[N+]1(C)CCC[C@H]1[C@H]1CS[C@@](O1)(C1CCCCC1)c1ccccc1 Show InChI InChI=1S/C21H32NOS/c1-22(2)15-9-14-19(22)20-16-24-21(23-20,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3,5-6,10-11,18-20H,4,7-9,12-16H2,1-2H3/q+1/t19-,20+,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Human immunodeficiency virus 1 reverse transcriptase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361262
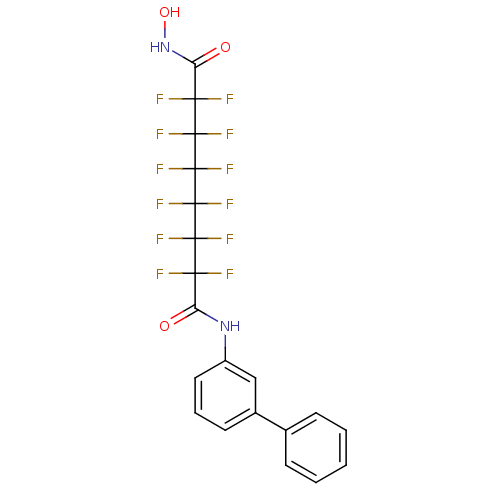
(CHEMBL1934904)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C20H12F12N2O3/c21-15(22,13(35)33-12-8-4-7-11(9-12)10-5-2-1-3-6-10)17(25,26)19(29,30)20(31,32)18(27,28)16(23,24)14(36)34-37/h1-9,37H,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411271

(CHEMBL5267112)Show SMILES Cc1cc(C)c2cccc(OCc3c(Cl)ccc(c3Cl)S(=O)(=O)NC3(CCCC3)C(=O)N3CCN(CC3)C(=O)[C@H](CCCC[N+](C)(C)C)[N+](C)(C)C)c2n1 Show InChI InChI=1S/C40H58Cl2N6O5S/c1-28-26-29(2)43-37-30(28)14-13-16-34(37)53-27-31-32(41)17-18-35(36(31)42)54(51,52)44-40(19-10-11-20-40)39(50)46-23-21-45(22-24-46)38(49)33(48(6,7)8)15-9-12-25-47(3,4)5/h13-14,16-18,26,33,44H,9-12,15,19-25,27H2,1-8H3/q+2/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to Human immunodeficiency virus 1 reverse transcriptase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361258
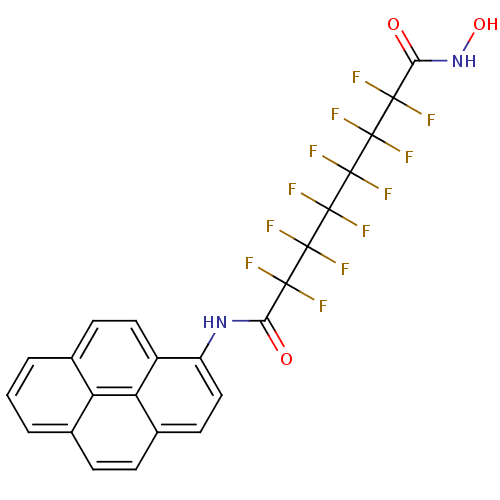
(CHEMBL1934900)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc2ccc3cccc4ccc1c2c34 Show InChI InChI=1S/C24H12F12N2O3/c25-19(26,21(29,30)23(33,34)24(35,36)22(31,32)20(27,28)18(40)38-41)17(39)37-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,41H,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50412091
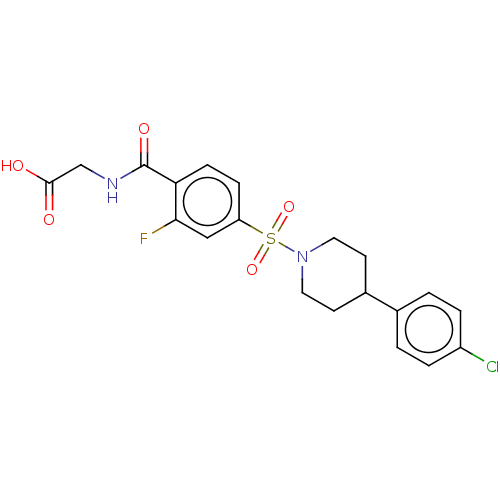
(CHEMBL5267510)Show SMILES COc1cc(cc(OC)c1OC)-c1cc2O[C@]3(C)CC[C@@]4(O)[C@@](C)(CI)C=CC(=O)[C@]4(C)[C@@]3(O)Cc2c(=O)o1 |r,c:27| Show InChI InChI=1S/C29H33IO9/c1-25(15-30)8-7-22(31)27(3)28(25,33)10-9-26(2)29(27,34)14-17-19(39-26)13-18(38-24(17)32)16-11-20(35-4)23(37-6)21(12-16)36-5/h7-8,11-13,33-34H,9-10,14-15H2,1-6H3/t25-,26-,27+,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR2 adult or last-instar nymphs homogenates assessed as bimolecular rate... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361250

(CHEMBL1934892)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C14H7ClF12N2O3/c15-5-1-3-6(4-2-5)28-7(30)9(16,17)11(20,21)13(24,25)14(26,27)12(22,23)10(18,19)8(31)29-32/h1-4,32H,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361254
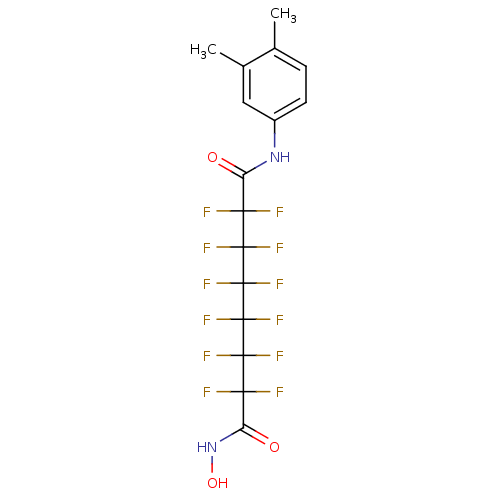
(CHEMBL1934896)Show SMILES Cc1ccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)NO)cc1C Show InChI InChI=1S/C16H12F12N2O3/c1-6-3-4-8(5-7(6)2)29-9(31)11(17,18)13(21,22)15(25,26)16(27,28)14(23,24)12(19,20)10(32)30-33/h3-5,33H,1-2H3,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411972

(CHEMBL5271929)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2N(C2CCCCCC2)C(=O)N(CC(=O)Nc2cccc(SCC(O)=O)c2)C1=O Show InChI InChI=1S/C31H38N4O6S/c1-31(2,3)26(36)18-33-24-15-8-9-16-25(24)35(22-12-6-4-5-7-13-22)30(41)34(29(33)40)19-27(37)32-21-11-10-14-23(17-21)42-20-28(38)39/h8-11,14-17,22H,4-7,12-13,18-20H2,1-3H3,(H,32,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361261
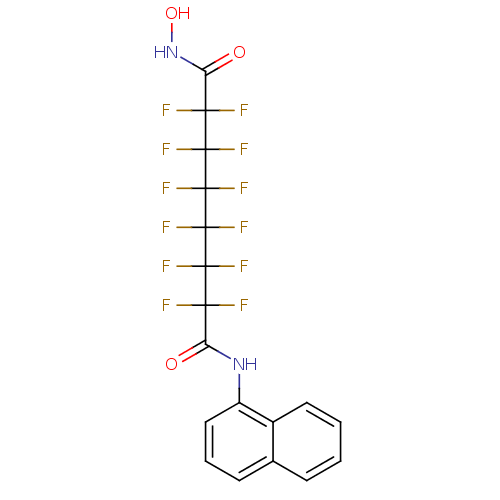
(CHEMBL1934903)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C18H10F12N2O3/c19-13(20,11(33)31-10-7-3-5-8-4-1-2-6-9(8)10)15(23,24)17(27,28)18(29,30)16(25,26)14(21,22)12(34)32-35/h1-7,35H,(H,31,33)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50412345
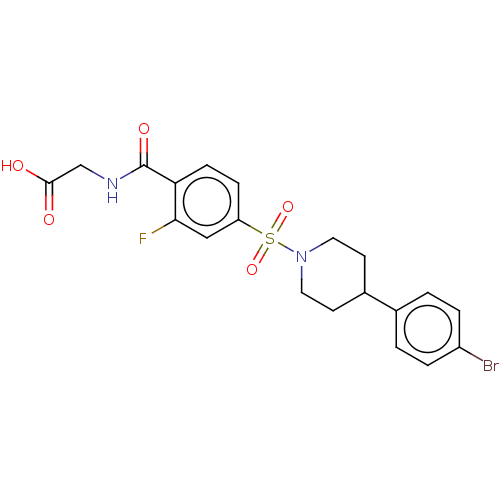
(CHEMBL5274579)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)NCc2cn3cc(C)sc3[n+]2Cc2ccc3ccccc3c2)cc1 |r| Show InChI InChI=1S/C38H37N5O5S/c1-24(2)48-36(46)29-12-14-31(15-13-29)40-37(47)41-34(19-26-9-16-33(44)17-10-26)35(45)39-20-32-23-42-21-25(3)49-38(42)43(32)22-27-8-11-28-6-4-5-7-30(28)18-27/h4-18,21,23-24,34H,19-20,22H2,1-3H3,(H3-,39,40,41,44,45,46,47)/p+1/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR2 adult or last-instar nymphs homogenates assessed as bimolecular rate... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50242196
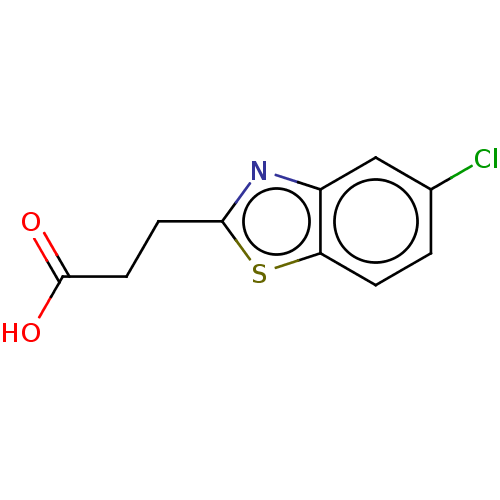
(CHEMBL4069423)Show InChI InChI=1S/C10H8ClNO2S/c11-6-1-2-8-7(5-6)12-9(15-8)3-4-10(13)14/h1-2,5H,3-4H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease in 5%DMSO |
J Med Chem 60: 9090-9096 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00933
BindingDB Entry DOI: 10.7270/Q2M32Z5T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361260
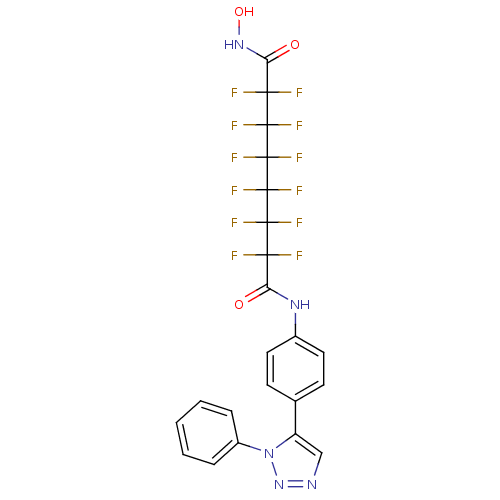
(CHEMBL1934902)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(cc1)-c1cnnn1-c1ccccc1 Show InChI InChI=1S/C22H13F12N5O3/c23-17(24,19(27,28)21(31,32)22(33,34)20(29,30)18(25,26)16(41)37-42)15(40)36-12-8-6-11(7-9-12)14-10-35-38-39(14)13-4-2-1-3-5-13/h1-10,42H,(H,36,40)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361255
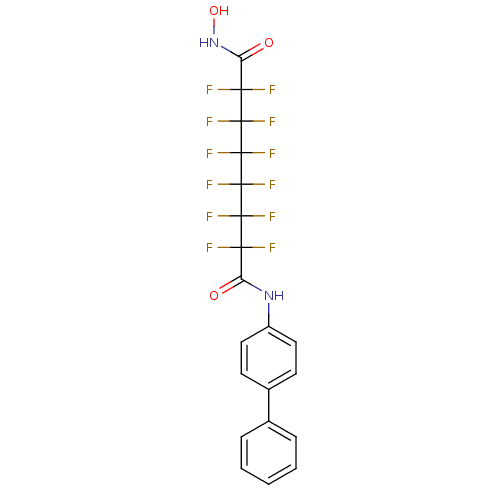
(CHEMBL1934897)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H12F12N2O3/c21-15(22,13(35)33-12-8-6-11(7-9-12)10-4-2-1-3-5-10)17(25,26)19(29,30)20(31,32)18(27,28)16(23,24)14(36)34-37/h1-9,37H,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50242196

(CHEMBL4069423)Show InChI InChI=1S/C10H8ClNO2S/c11-6-1-2-8-7(5-6)12-9(15-8)3-4-10(13)14/h1-2,5H,3-4H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal Avi-tagged/C-terminal His6-tagged HDAC6 zinc-finger ubiquitin binding domain (1109 to 1215 residues)(unknown origin) e... |
J Med Chem 60: 9090-9096 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00933
BindingDB Entry DOI: 10.7270/Q2M32Z5T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411355

(CHEMBL5282155)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(CC)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)NC(C)(C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C44H69N11O11S2/c1-8-24(5)36-42(65)50-27(14-15-32(45)56)38(61)51-30(20-33(46)57)39(62)52-31(41(64)55-44(6,7)43(66)53-28(18-23(3)4)37(60)48-21-34(47)58)22-68-67-17-16-35(59)49-29(40(63)54-36)19-26-12-10-25(9-2)11-13-26/h10-13,23-24,27-31,36H,8-9,14-22H2,1-7H3,(H2,45,56)(H2,46,57)(H2,47,58)(H,48,60)(H,49,59)(H,50,65)(H,51,61)(H,52,62)(H,53,66)(H,54,63)(H,55,64)/t24-,27-,28-,29-,30-,31-,36?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411977

(CHEMBL5280865)Show SMILES CNc1cccc(NC(=O)CN2C(=O)N(CC(=O)C(C)(C)C)c3ccccc3N(C3CCCCC3)C2=O)c1 Show InChI InChI=1S/C29H37N5O4/c1-29(2,3)25(35)18-32-23-15-8-9-16-24(23)34(22-13-6-5-7-14-22)28(38)33(27(32)37)19-26(36)31-21-12-10-11-20(17-21)30-4/h8-12,15-17,22,30H,5-7,13-14,18-19H2,1-4H3,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361252
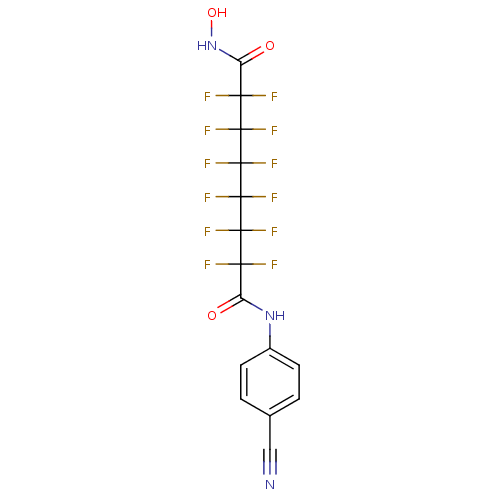
(CHEMBL1934894)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc(cc1)C#N Show InChI InChI=1S/C15H7F12N3O3/c16-10(17,8(31)29-7-3-1-6(5-28)2-4-7)12(20,21)14(24,25)15(26,27)13(22,23)11(18,19)9(32)30-33/h1-4,33H,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
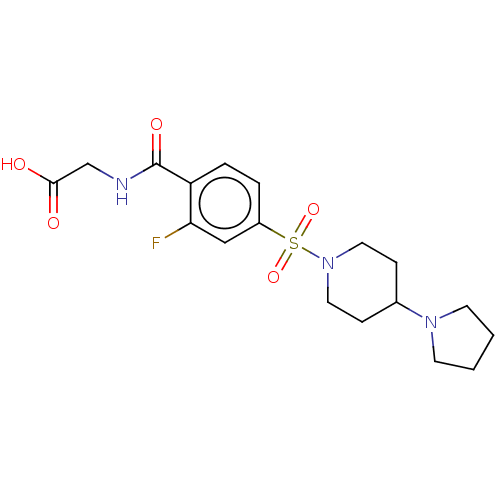
(Homo sapiens (Human)) | BDBM50412346
(CHEMBL5283754)Show SMILES Cc1cn2cc(CNC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)Nc3ccc(s3)C(=O)OC3CCCCC3)[n+](Cc3ccc4ccccc4c3)c2s1 |r| Show InChI InChI=1S/C39H39N5O5S2/c1-25-22-43-24-30(44(39(43)50-25)23-27-11-14-28-7-5-6-8-29(28)19-27)21-40-36(46)33(20-26-12-15-31(45)16-13-26)41-38(48)42-35-18-17-34(51-35)37(47)49-32-9-3-2-4-10-32/h5-8,11-19,22,24,32-33H,2-4,9-10,20-21,23H2,1H3,(H3-,40,41,42,45,46,47,48)/p+1/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR2 adult or last-instar nymphs homogenates assessed as bimolecular rate... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
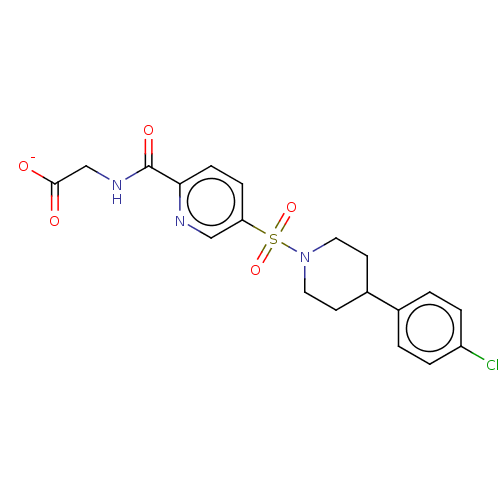
(Homo sapiens (Human)) | BDBM50412353
(CHEMBL5266862)Show SMILES CCCCc1nc2cc(NC(=O)c3ccccc3Cl)ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H28ClN3O3/c1-2-3-12-30-35-28-19-23(34-31(37)26-10-6-7-11-27(26)33)17-18-29(28)36(30)20-21-13-15-22(16-14-21)24-8-4-5-9-25(24)32(38)39/h4-11,13-19H,2-3,12,20H2,1H3,(H,34,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR1 adult or last-instar nymphs homogenates assessed as bimolecular rate... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
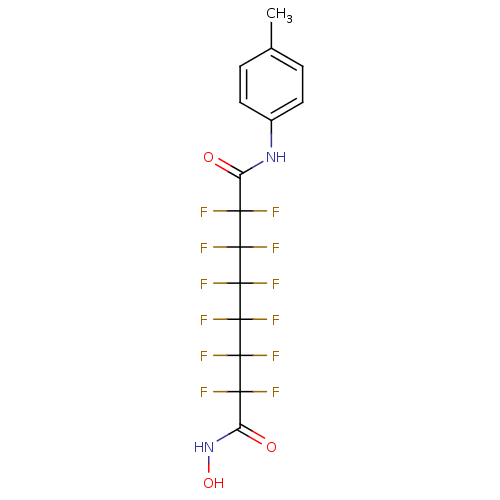
(Homo sapiens (Human)) | BDBM50361249
(CHEMBL1934891)Show SMILES Cc1ccc(NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)NO)cc1 Show InChI InChI=1S/C15H10F12N2O3/c1-6-2-4-7(5-3-6)28-8(30)10(16,17)12(20,21)14(24,25)15(26,27)13(22,23)11(18,19)9(31)29-32/h2-5,32H,1H3,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
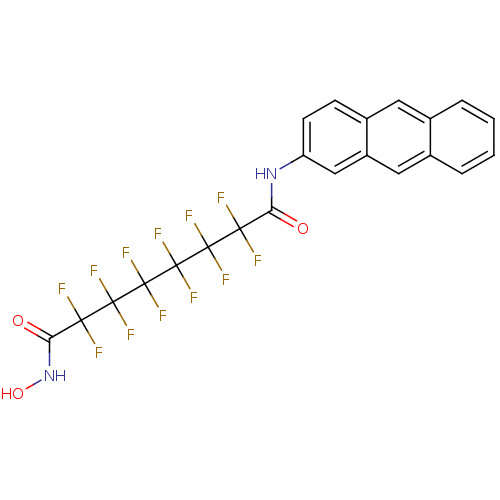
(Homo sapiens (Human)) | BDBM50361265
(CHEMBL1934907)Show SMILES ONC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccc2cc3ccccc3cc2c1 Show InChI InChI=1S/C22H12F12N2O3/c23-17(24,19(27,28)21(31,32)22(33,34)20(29,30)18(25,26)16(38)36-39)15(37)35-14-6-5-12-7-10-3-1-2-4-11(10)8-13(12)9-14/h1-9,39H,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50361268
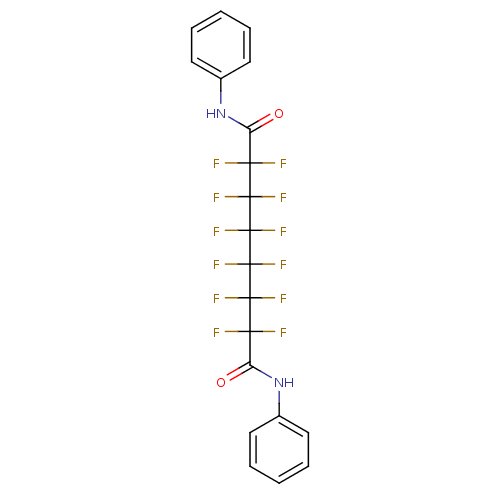
(CHEMBL1934910)Show SMILES FC(F)(C(=O)Nc1ccccc1)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H12F12N2O2/c21-15(22,13(35)33-11-7-3-1-4-8-11)17(25,26)19(29,30)20(31,32)18(27,28)16(23,24)14(36)34-12-9-5-2-6-10-12/h1-10H,(H,33,35)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
University of Applied Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Boc-L-Lys(acetyl)-MCA as substrate by fluorogenic enzymatic assay |
Bioorg Med Chem 20: 985-95 (2012)
Article DOI: 10.1016/j.bmc.2011.11.041
BindingDB Entry DOI: 10.7270/Q2QJ7HRC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50412353

(CHEMBL5266862)Show SMILES CCCCc1nc2cc(NC(=O)c3ccccc3Cl)ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H28ClN3O3/c1-2-3-12-30-35-28-19-23(34-31(37)26-10-6-7-11-27(26)33)17-18-29(28)36(30)20-21-13-15-22(16-14-21)24-8-4-5-9-25(24)32(38)39/h4-11,13-19H,2-3,12,20H2,1H3,(H,34,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-susceptible clone of Schizaphis graminum OSS adult or last-instar nymphs homogenates assessed as bimolecular ra... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50412352

(CHEMBL5278336)Show SMILES CCCCc1nc2cc(NC(=O)c3ccc(Cl)cc3)ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H28ClN3O3/c1-2-3-8-30-35-28-19-25(34-31(37)23-13-15-24(33)16-14-23)17-18-29(28)36(30)20-21-9-11-22(12-10-21)26-6-4-5-7-27(26)32(38)39/h4-7,9-19H,2-3,8,20H2,1H3,(H,34,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in organophosphate-resistant clone of Schizaphis graminum OR2 adult or last-instar nymphs homogenates assessed as bimolecular rate... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411432
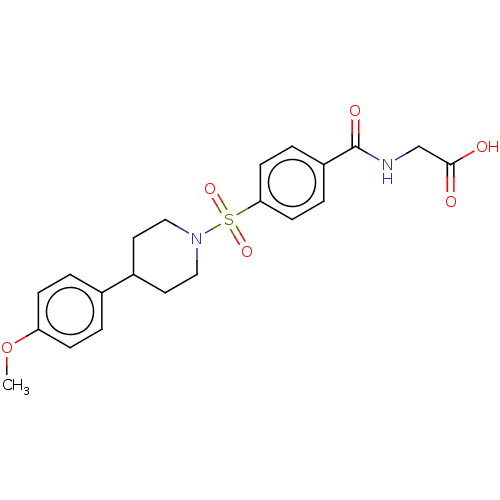
(CHEMBL5266727)Show SMILES Cc1sc(CN2CCNCC2)cc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.22.23,THB:22:21:18:24.23.25,22:23:20.21.26:18,25:23:20:26.17.18,25:17:20:24.22.23| Show InChI InChI=1S/C22H33N3OS/c1-15-20(9-19(27-15)13-25-4-2-23-3-5-25)21(26)24-14-22-10-16-6-17(11-22)8-18(7-16)12-22/h9,16-18,23H,2-8,10-14H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411971
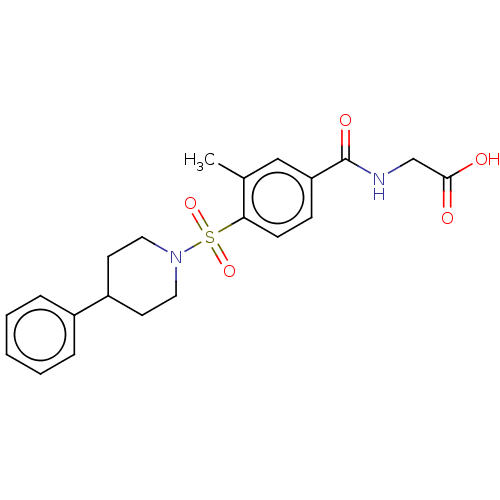
(CHEMBL5289065)Show SMILES CN(c1nnn[nH]1)c1cccc(NC(=O)CN2C(=O)N(CC(=O)C(C)(C)C)c3ccccc3N(C3CCCCC3)C2=O)c1 Show InChI InChI=1S/C30H37N9O4/c1-30(2,3)25(40)18-37-23-15-8-9-16-24(23)39(21-12-6-5-7-13-21)29(43)38(28(37)42)19-26(41)31-20-11-10-14-22(17-20)36(4)27-32-34-35-33-27/h8-11,14-17,21H,5-7,12-13,18-19H2,1-4H3,(H,31,41)(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411973
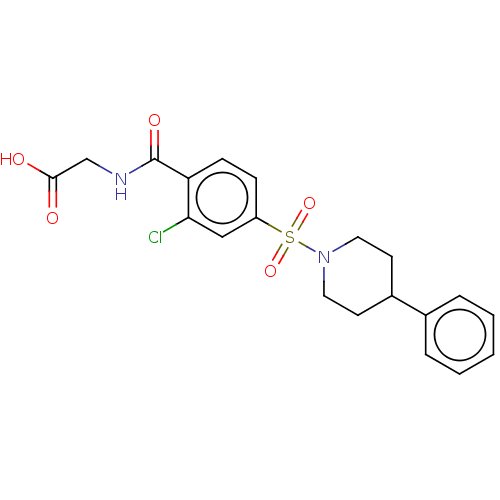
(CHEMBL5286558)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2N(C2CCCCCC2)C(=O)N(CC(=O)Nc2cccc(CC(O)=O)c2)C1=O Show InChI InChI=1S/C31H38N4O6/c1-31(2,3)26(36)19-33-24-15-8-9-16-25(24)35(23-13-6-4-5-7-14-23)30(41)34(29(33)40)20-27(37)32-22-12-10-11-21(17-22)18-28(38)39/h8-12,15-17,23H,4-7,13-14,18-20H2,1-3H3,(H,32,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411434

(CHEMBL5281654)Show SMILES Clc1c(CN2CCCCC2)cccc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:17:18:21:25.23.24,THB:23:22:19:25.24.26,23:24:21.22.27:19,26:24:21:27.18.19,26:18:21:25.23.24| Show InChI InChI=1S/C24H33ClN2O/c25-22-20(15-27-7-2-1-3-8-27)5-4-6-21(22)23(28)26-16-24-12-17-9-18(13-24)11-19(10-17)14-24/h4-6,17-19H,1-3,7-16H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411964
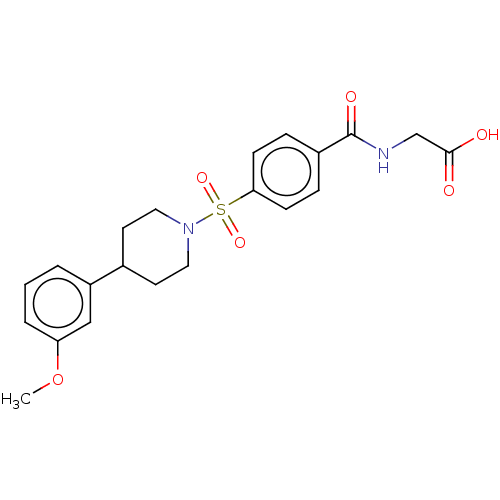
(CHEMBL5290467)Show SMILES [H]C12CCN(CC1)C[C@]2(OC)C#C[C@@](O)(C1CCCCCC1)c1ccccc1 |wU:13.15,8.10,wD:13.24,8.12,TLB:11:8:3.2:5.6,THB:9:8:3.2:5.6,(-2.17,-8.46,;-1.07,-9.56,;.28,-10.17,;.56,-11.56,;-.81,-10.93,;-.56,-9.03,;-1,-7.93,;-2.35,-11.59,;-2.54,-10.21,;-3.33,-8.87,;-4.87,-8.88,;-3.64,-11.29,;-4.74,-12.38,;-5.83,-13.46,;-6.93,-14.54,;-6.92,-12.37,;-8.37,-12.86,;-9.68,-12.04,;-9.85,-10.51,;-8.75,-9.31,;-7.22,-9.61,;-6.41,-10.91,;-4.75,-14.56,;-5.16,-16.04,;-4.08,-17.13,;-2.59,-16.74,;-2.18,-15.25,;-3.27,-14.16,)| Show InChI InChI=1S/C24H33NO2/c1-27-23(19-25-17-13-20(23)14-18-25)15-16-24(26,22-11-7-4-8-12-22)21-9-5-2-3-6-10-21/h4,7-8,11-12,20-21,26H,2-3,5-6,9-10,13-14,17-19H2,1H3/t23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411363

(CHEMBL5277702)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]C([#6])([#6])[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C83H140N32O21/c1-45(67(124)106-54(27-17-35-97-80(91)92)70(127)107-51(24-11-14-32-84)69(126)110-55(28-18-36-98-81(93)94)71(128)108-52(25-12-15-33-85)72(129)111-58(40-61(89)119)75(132)105-50(66(90)123)30-31-60(88)118)103-77(134)59(44-116)112-73(130)53(26-13-16-34-86)109-74(131)56(29-19-37-99-82(95)96)113-79(136)83(3,4)115-64(122)43-102-78(135)65(46(2)117)114-76(133)57(39-48-22-9-6-10-23-48)104-63(121)42-100-62(120)41-101-68(125)49(87)38-47-20-7-5-8-21-47/h5-10,20-23,45-46,49-59,65,116-117H,11-19,24-44,84-87H2,1-4H3,(H2,88,118)(H2,89,119)(H2,90,123)(H,100,120)(H,101,125)(H,102,135)(H,103,134)(H,104,121)(H,105,132)(H,106,124)(H,107,127)(H,108,128)(H,109,131)(H,110,126)(H,111,129)(H,112,130)(H,113,136)(H,114,133)(H,115,122)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99)/t45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50411976
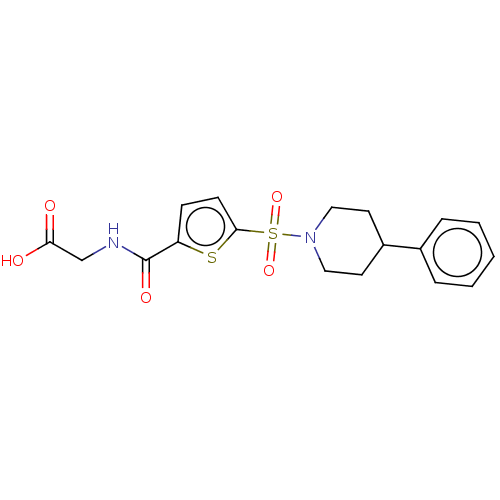
(CHEMBL5273795)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2N(C2CCCCC2)C(=O)N(CC(=O)Nc2cccc(CCC(O)=O)c2)C1=O Show InChI InChI=1S/C31H38N4O6/c1-31(2,3)26(36)19-33-24-14-7-8-15-25(24)35(23-12-5-4-6-13-23)30(41)34(29(33)40)20-27(37)32-22-11-9-10-21(18-22)16-17-28(38)39/h7-11,14-15,18,23H,4-6,12-13,16-17,19-20H2,1-3H3,(H,32,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]angiotensin 2 from type-1 angiotensin 2 receptor in Bos taurus (bovine) adrenal cortical membrane |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data