 Found 12 hits of ki data for polymerid = 50004805
Found 12 hits of ki data for polymerid = 50004805 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311
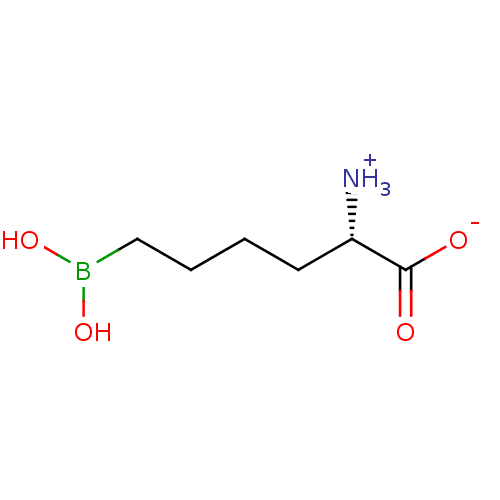
(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drexel University
Curated by ChEMBL
| Assay Description
Binding affinity to human arginase 2 |
J Med Chem 54: 5432-43 (2011)
Article DOI: 10.1021/jm200443b
BindingDB Entry DOI: 10.7270/Q2TH8N21 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311

(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561046

(CHEMBL4790798) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561045

(CHEMBL4758805)Show SMILES NC(CCCCB(O)O)(CCCN1CCC(O)(CC1)c1ccc(Cl)cc1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034
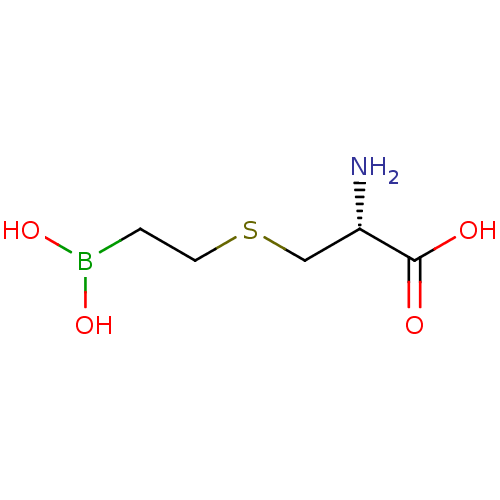
(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099
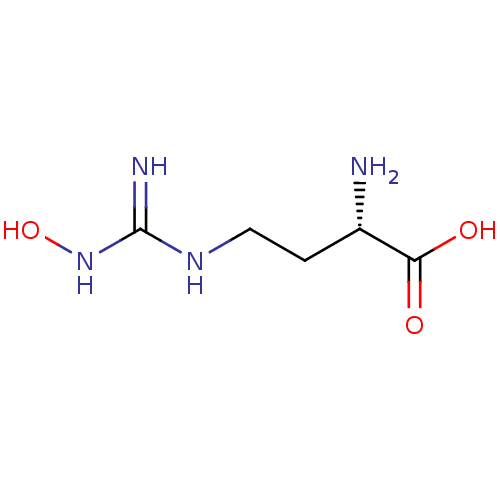
(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099

(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of arginase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2017.12.044
BindingDB Entry DOI: 10.7270/Q2736THR |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50294581
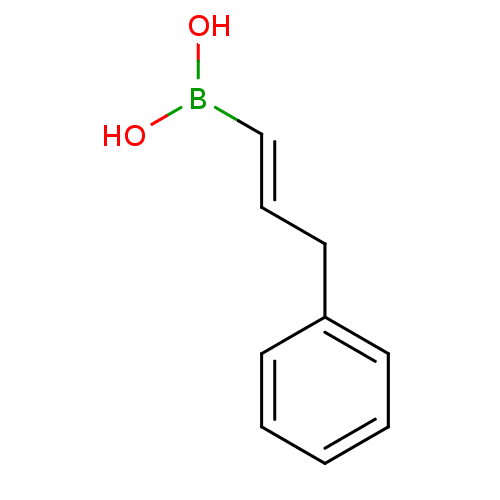
(3-phenylprop-1-enylboronic acid | CHEMBL539140)Show InChI InChI=1S/C9H11BO2/c11-10(12)8-4-7-9-5-2-1-3-6-9/h1-6,8,11-12H,7H2/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034

(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50230418
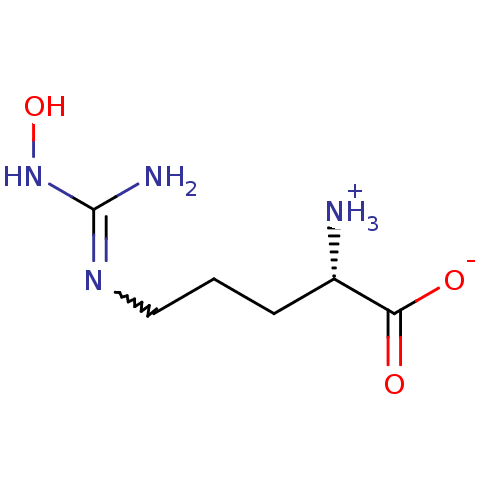
(CHEMBL260629 | N(gamma)-hydroxy-L-arginine | N-OME...)Show SMILES NC(NO)=NCCC[C@H]([NH3+])C([O-])=O |r,w:4.4| Show InChI InChI=1S/C6H14N4O3/c7-4(5(11)12)2-1-3-9-6(8)10-13/h4,13H,1-3,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 at pH 7.5 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462601
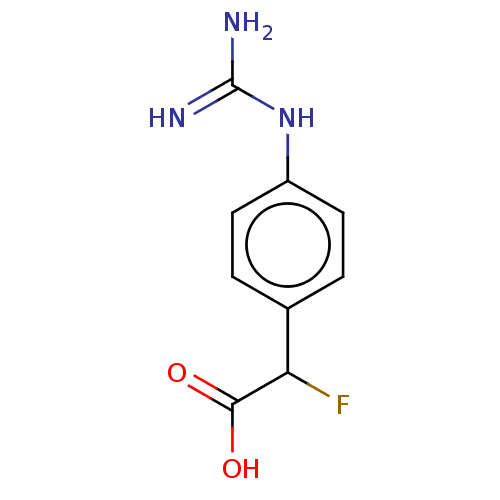
(CHEMBL4250607)Show InChI InChI=1S/C9H10FN3O2/c10-7(8(14)15)5-1-3-6(4-2-5)13-9(11)12/h1-4,7H,(H,14,15)(H4,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462600
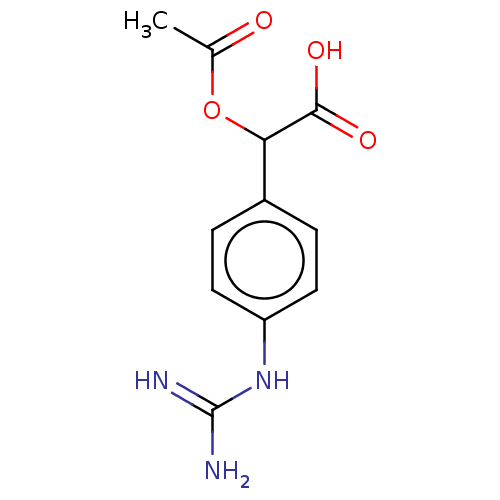
(CHEMBL4238387)Show InChI InChI=1S/C11H13N3O4/c1-6(15)18-9(10(16)17)7-2-4-8(5-3-7)14-11(12)13/h2-5,9H,1H3,(H,16,17)(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data