 Found 12969 hits Enz. Inhib. hit(s) with Target = 'Glycogen synthase kinase 3'
Found 12969 hits Enz. Inhib. hit(s) with Target = 'Glycogen synthase kinase 3' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50491919
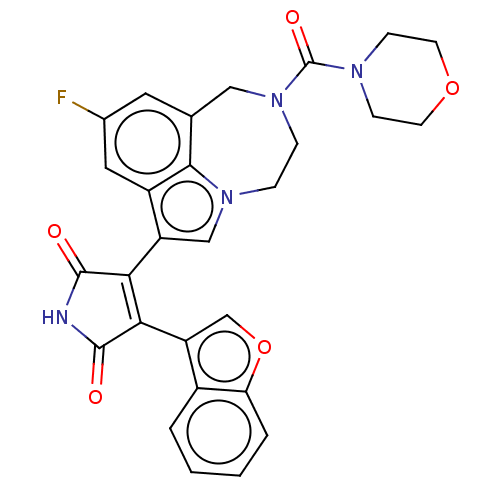
(CHEMBL2386094)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4coc5ccccc45)c(c1)c23)C(=O)N1CCOCC1 |t:11| Show InChI InChI=1S/C28H23FN4O5/c29-17-11-16-13-33(28(36)31-7-9-37-10-8-31)6-5-32-14-20(19(12-17)25(16)32)23-24(27(35)30-26(23)34)21-15-38-22-4-2-1-3-18(21)22/h1-4,11-12,14-15H,5-10,13H2,(H,30,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta-mediated YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE phosphorylation |
J Med Chem 56: 5115-29 (2013)
Article DOI: 10.1021/jm400511s
BindingDB Entry DOI: 10.7270/Q21R6TFJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610391
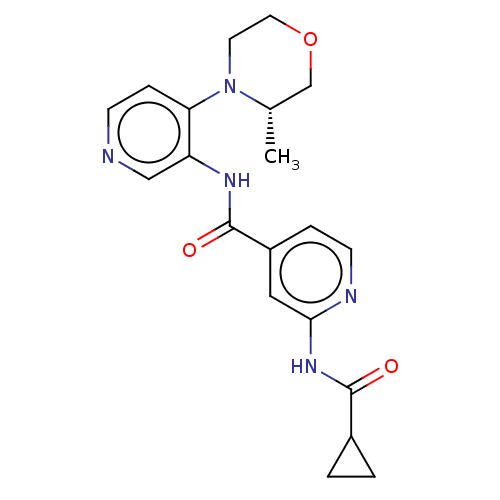
(CHEMBL5290966)Show SMILES C[C@H]1COCCN1c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463
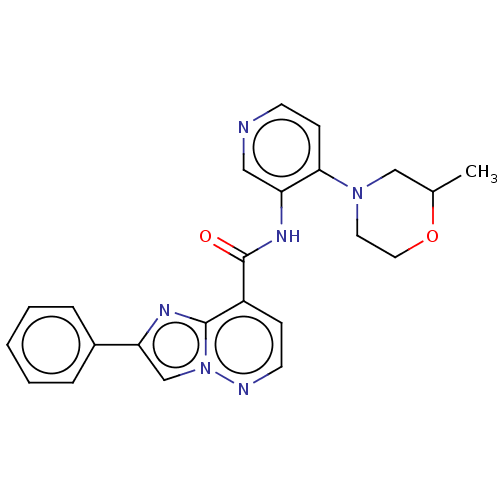
(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610453
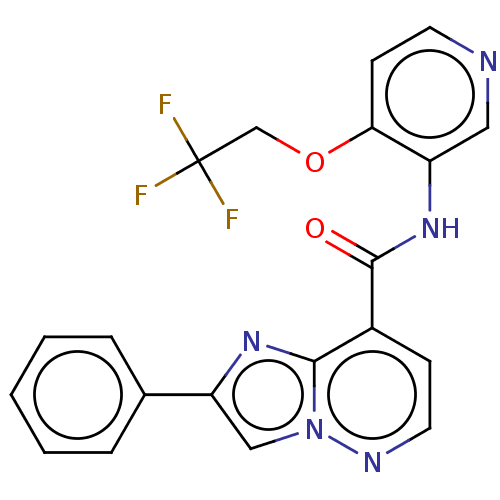
(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM251572
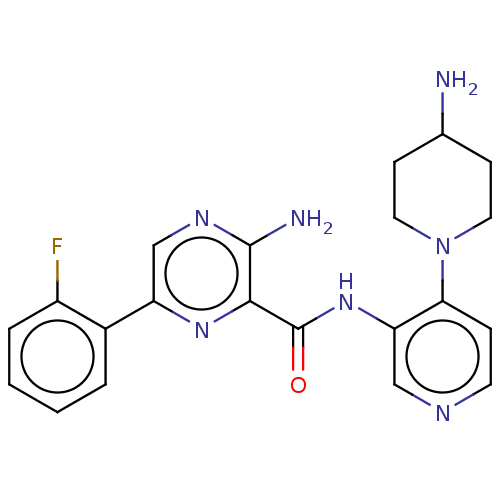
(US9452998, Table 4 Compound 2)Show SMILES NC1CCN(CC1)c1ccncc1NC(=O)c1nc(cnc1N)-c1ccccc1F Show InChI InChI=1S/C21H22FN7O/c22-15-4-2-1-3-14(15)16-12-26-20(24)19(27-16)21(30)28-17-11-25-8-5-18(17)29-9-6-13(23)7-10-29/h1-5,8,11-13H,6-7,9-10,23H2,(H2,24,26)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... |
US Patent US9452998 (2016)
BindingDB Entry DOI: 10.7270/Q2C82870 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610453

(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610459

(N-(4-(4-Cyanopiperidin-1-yl)pyridin-3-yl)-2-phenyl...)Show SMILES O=C(Nc1cnccc1N1CCC(CC1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610486

(2-(Cyclohex-1-en-1-yl)-N-(4-(4,4-difluoropiperidin...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1=CCCCC1 |t:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610384
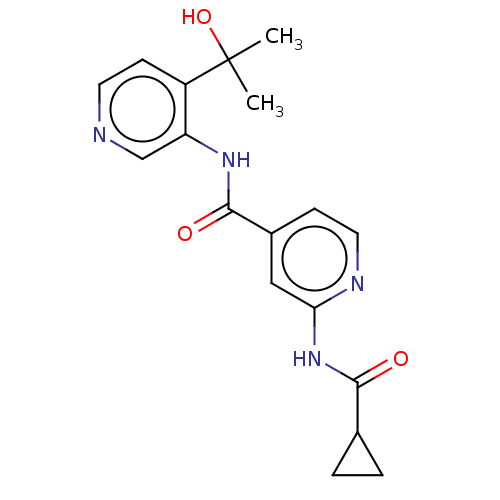
(CHEMBL5268011)Show SMILES CC(C)(O)c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50613245

(CHEMBL5266553)Show SMILES C[C@H]1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269429
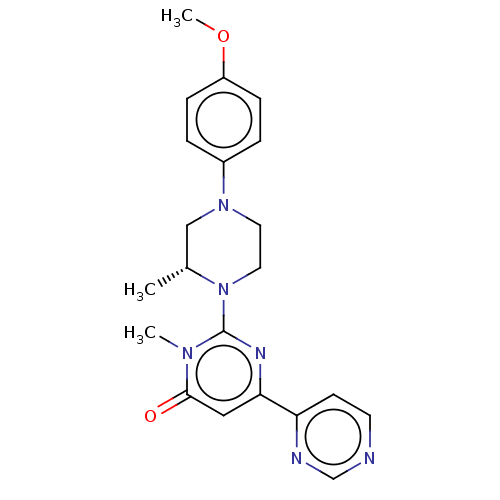
(CHEMBL4084855)Show SMILES COc1ccc(cc1)N1CCN([C@H](C)C1)c1nc(cc(=O)n1C)-c1ccncn1 |r| Show InChI InChI=1S/C21H24N6O2/c1-15-13-26(16-4-6-17(29-3)7-5-16)10-11-27(15)21-24-19(12-20(28)25(21)2)18-8-9-22-14-23-18/h4-9,12,14-15H,10-11,13H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... |
Bioorg Med Chem Lett 27: 3733-3738 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.077
BindingDB Entry DOI: 10.7270/Q2XW4N9C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50293306

(3-(5-methoxybenzofuran-7-yl)-4-(1-(2-(4-methylpipe...)Show SMILES COc1cc(C2=C(C(=O)NC2=O)c2cn(CCN3CCN(C)CC3)c3ccccc23)c2occc2c1 |t:5| Show InChI InChI=1S/C28H28N4O4/c1-30-8-10-31(11-9-30)12-13-32-17-22(20-5-3-4-6-23(20)32)25-24(27(33)29-28(25)34)21-16-19(35-2)15-18-7-14-36-26(18)21/h3-7,14-17H,8-13H2,1-2H3,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Road
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta |
Eur J Med Chem 44: 2361-71 (2009)
Article DOI: 10.1016/j.ejmech.2008.08.012
BindingDB Entry DOI: 10.7270/Q2057FZX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610396
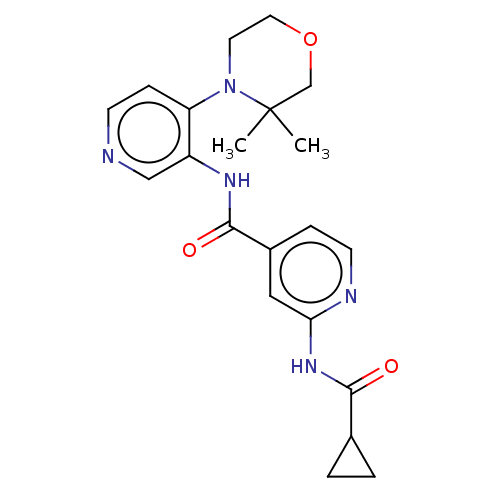
(CHEMBL5278104)Show SMILES CC1(C)COCCN1c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610428
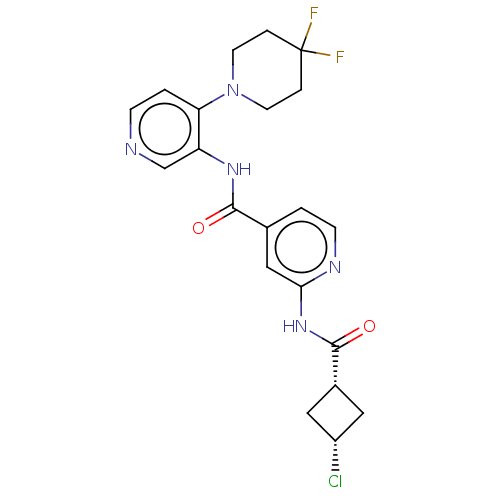
(CHEMBL5284020)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnc(NC(=O)[C@@H]2C[C@H](Cl)C2)c1 |r,wU:25.26,27.29,(4.1,-2.55,;3.33,-1.21,;2.56,-2.55,;2,-.44,;2,1.1,;3.33,1.87,;4.67,1.1,;4.67,-.44,;3.33,3.41,;4.66,4.18,;4.66,5.72,;3.33,6.49,;2,5.72,;2,4.18,;.66,3.41,;-.67,4.18,;-.67,5.72,;-2.01,3.41,;-3.33,4.18,;-4.67,3.41,;-4.67,1.86,;-3.33,1.1,;-3.33,-.44,;-2,-1.21,;-.66,-.44,;-2,-2.75,;-3.09,-3.84,;-1.98,-4.95,;-1.98,-6.49,;-.89,-3.86,;-2.01,1.87,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50613418
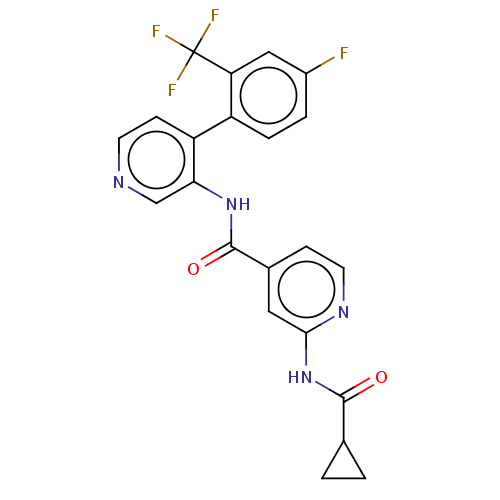
(CHEMBL5268185)Show SMILES Fc1ccc(-c2ccncc2NC(=O)c2ccnc(NC(=O)C3CC3)c2)c(c1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610483

(2-Cyclopropyl-N-(4-(4,4-difluoropiperidin-1-yl)pyr...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610389
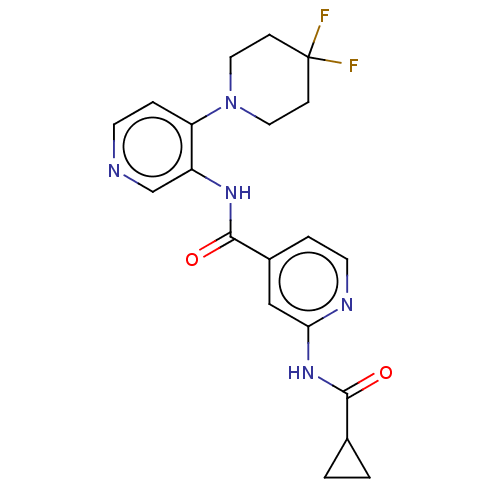
(CHEMBL5282887)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM251574

(US9452998, Table 4 Compound 4)Show SMILES NC1CCN(CC1)c1ccncc1NC(=O)c1nc(cnc1N)-c1ccccc1 Show InChI InChI=1S/C21H23N7O/c22-15-7-10-28(11-8-15)18-6-9-24-12-17(18)27-21(29)19-20(23)25-13-16(26-19)14-4-2-1-3-5-14/h1-6,9,12-13,15H,7-8,10-11,22H2,(H2,23,25)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... |
US Patent US9452998 (2016)
BindingDB Entry DOI: 10.7270/Q2C82870 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610483

(2-Cyclopropyl-N-(4-(4,4-difluoropiperidin-1-yl)pyr...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM251576

(US9452998, Table 4 Compound 6)Show SMILES Nc1ncc(nc1C(=O)Nc1cnccc1N1CCNCC1)-c1ccccc1F Show InChI InChI=1S/C20H20FN7O/c21-14-4-2-1-3-13(14)15-12-25-19(22)18(26-15)20(29)27-16-11-24-6-5-17(16)28-9-7-23-8-10-28/h1-6,11-12,23H,7-10H2,(H2,22,25)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... |
US Patent US9452998 (2016)
BindingDB Entry DOI: 10.7270/Q2C82870 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610458
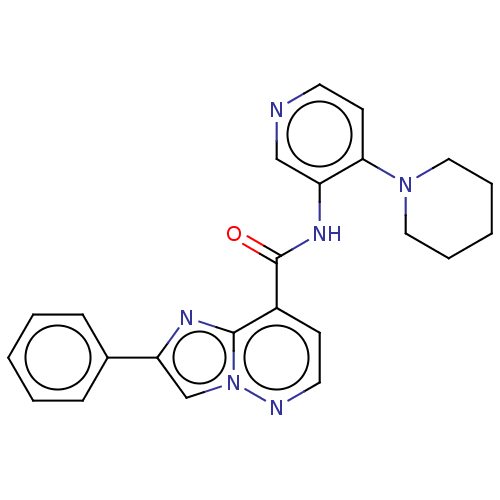
(2-Phenyl-N-(4-(piperidin-1-yl)pyridin-3-yl)imidazo...)Show SMILES O=C(Nc1cnccc1N1CCCCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610459

(N-(4-(4-Cyanopiperidin-1-yl)pyridin-3-yl)-2-phenyl...)Show SMILES O=C(Nc1cnccc1N1CCC(CC1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610460
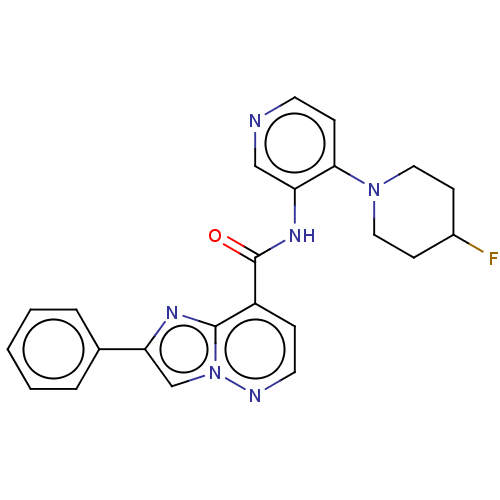
(N-(4-(4-Fluoropiperidin-1-yl)pyridin-3-yl)-2-pheny...)Show SMILES FC1CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610461

(N-(4-(4,4-Difluoropiperidin-1-yl)pyridin-3-yl)-2-p...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610462
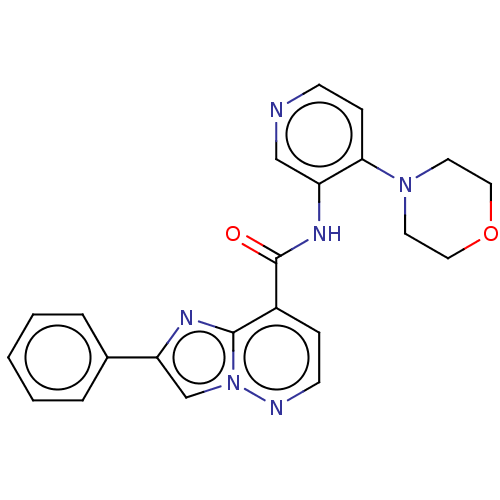
(N-(4-Morpholinopyridin-3-yl)-2-phenylimidazo[1,2-b...)Show SMILES O=C(Nc1cnccc1N1CCOCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50491937

(CHEMBL2386090)Show SMILES O=C(N1CCn2cc(C3=C(C(=O)NC3=O)c3coc4ccccc34)c3cccc(C1)c23)c1cnccn1 |t:8| Show InChI InChI=1S/C28H19N5O4/c34-26-23(24(27(35)31-26)20-15-37-22-7-2-1-5-17(20)22)19-14-32-10-11-33(28(36)21-12-29-8-9-30-21)13-16-4-3-6-18(19)25(16)32/h1-9,12,14-15H,10-11,13H2,(H,31,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta-mediated YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE phosphorylation |
J Med Chem 56: 5115-29 (2013)
Article DOI: 10.1021/jm400511s
BindingDB Entry DOI: 10.7270/Q21R6TFJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269428
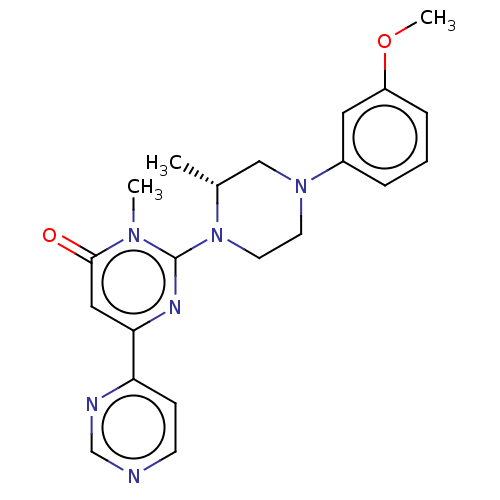
(CHEMBL4063206)Show SMILES COc1cccc(c1)N1CCN([C@H](C)C1)c1nc(cc(=O)n1C)-c1ccncn1 |r| Show InChI InChI=1S/C21H24N6O2/c1-15-13-26(16-5-4-6-17(11-16)29-3)9-10-27(15)21-24-19(12-20(28)25(21)2)18-7-8-22-14-23-18/h4-8,11-12,14-15H,9-10,13H2,1-3H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... |
Bioorg Med Chem Lett 27: 3733-3738 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.077
BindingDB Entry DOI: 10.7270/Q2XW4N9C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269437
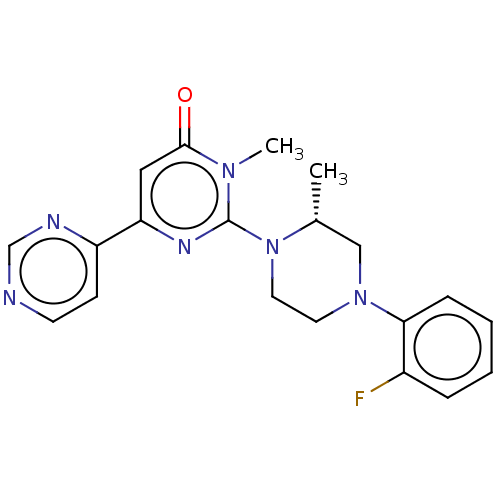
(CHEMBL4077376)Show SMILES C[C@@H]1CN(CCN1c1nc(cc(=O)n1C)-c1ccncn1)c1ccccc1F |r| Show InChI InChI=1S/C20H21FN6O/c1-14-12-26(18-6-4-3-5-15(18)21)9-10-27(14)20-24-17(11-19(28)25(20)2)16-7-8-22-13-23-16/h3-8,11,13-14H,9-10,12H2,1-2H3/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... |
Bioorg Med Chem Lett 27: 3733-3738 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.077
BindingDB Entry DOI: 10.7270/Q2XW4N9C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610473
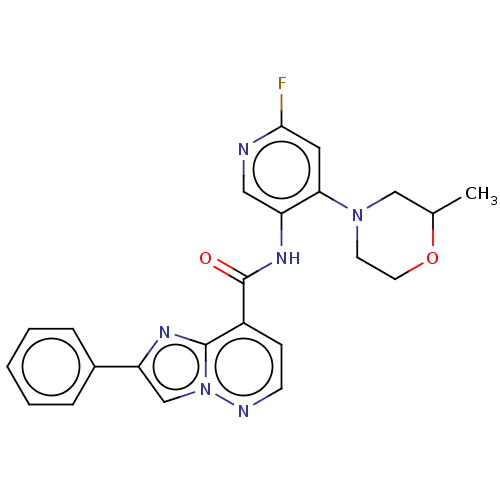
(N-(6-Fluoro-4-morpholinopyridin-3-yl)-2-phenylimid...)Show SMILES CC1CN(CCO1)c1cc(F)ncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610477
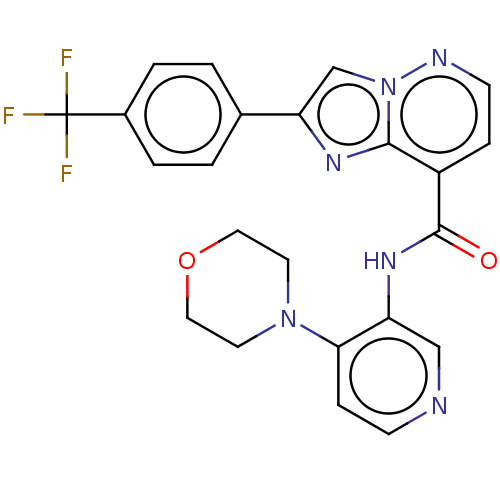
(N-(4-Morpholinopyridin-3-yl)-2-(4-(trifluoromethyl...)Show SMILES FC(F)(F)c1ccc(cc1)-c1cn2nccc(C(=O)Nc3cnccc3N3CCOCC3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM251575
(US9452998, Table 4 Compound 5)Show SMILES COc1ccc(cc1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC(N)CC1 Show InChI InChI=1S/C22H25N7O2/c1-31-16-4-2-14(3-5-16)17-13-26-21(24)20(27-17)22(30)28-18-12-25-9-6-19(18)29-10-7-15(23)8-11-29/h2-6,9,12-13,15H,7-8,10-11,23H2,1H3,(H2,24,26)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.0 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Types of GSK-3 assay used to test the selectivity/off target potential compounds of the invention with respect to PKC α/θ inhibition activity inclu... |
US Patent US9452998 (2016)
BindingDB Entry DOI: 10.7270/Q2C82870 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610462

(N-(4-Morpholinopyridin-3-yl)-2-phenylimidazo[1,2-b...)Show SMILES O=C(Nc1cnccc1N1CCOCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610461

(N-(4-(4,4-Difluoropiperidin-1-yl)pyridin-3-yl)-2-p...)Show SMILES FC1(F)CCN(CC1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610477

(N-(4-Morpholinopyridin-3-yl)-2-(4-(trifluoromethyl...)Show SMILES FC(F)(F)c1ccc(cc1)-c1cn2nccc(C(=O)Nc3cnccc3N3CCOCC3)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267760
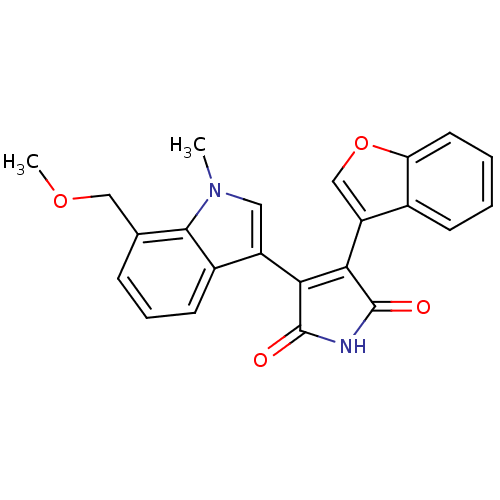
(3-Benzofuran-3-yl-4-(7-methoxymethyl-1-methyl-1H-i...)Show SMILES COCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2ccccc12 |t:15| Show InChI InChI=1S/C23H18N2O4/c1-25-10-16(15-8-5-6-13(11-28-2)21(15)25)19-20(23(27)24-22(19)26)17-12-29-18-9-4-3-7-14(17)18/h3-10,12H,11H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610458

(2-Phenyl-N-(4-(piperidin-1-yl)pyridin-3-yl)imidazo...)Show SMILES O=C(Nc1cnccc1N1CCCCC1)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269447

(CHEMBL4076186)Show SMILES Cl.COc1cc(F)ccc1C1CN(CCN1)c1nc(cc(=O)n1C)-c1ccncn1 Show InChI InChI=1S/C20H21FN6O2.ClH/c1-26-19(28)10-16(15-5-6-22-12-24-15)25-20(26)27-8-7-23-17(11-27)14-4-3-13(21)9-18(14)29-2;/h3-6,9-10,12,17,23H,7-8,11H2,1-2H3;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... |
Bioorg Med Chem Lett 27: 3726-3732 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.078
BindingDB Entry DOI: 10.7270/Q2T43WKZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50610383

(CHEMBL5283410) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50269447

(CHEMBL4076186)Show SMILES Cl.COc1cc(F)ccc1C1CN(CCN1)c1nc(cc(=O)n1C)-c1ccncn1 Show InChI InChI=1S/C20H21FN6O2.ClH/c1-26-19(28)10-16(15-5-6-22-12-24-15)25-20(26)27-8-7-23-17(11-27)14-4-3-13(21)9-18(14)29-2;/h3-6,9-10,12,17,23H,7-8,11H2,1-2H3;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... |
Bioorg Med Chem Lett 27: 3726-3732 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.078
BindingDB Entry DOI: 10.7270/Q2T43WKZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50613417
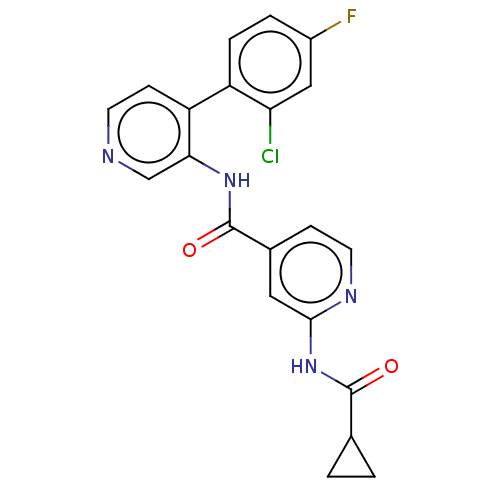
(CHEMBL5290300)Show SMILES Fc1ccc(c(Cl)c1)-c1ccncc1NC(=O)c1ccnc(NC(=O)C2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074676
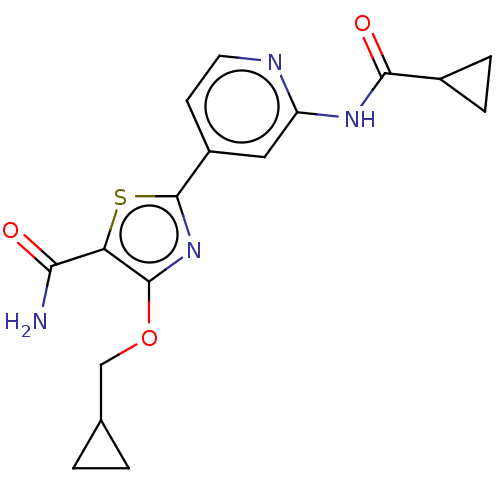
(CHEMBL3410091)Show SMILES NC(=O)c1sc(nc1OCC1CC1)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C17H18N4O3S/c18-14(22)13-16(24-8-9-1-2-9)21-17(25-13)11-5-6-19-12(7-11)20-15(23)10-3-4-10/h5-7,9-10H,1-4,8H2,(H2,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM118096

(US8664219, I-16 | US8664219, I-8)Show InChI InChI=1S/C18H21FN6/c1-11-8-15(22-16(21-11)12-6-4-2-3-5-7-12)23-18-14-9-13(19)10-20-17(14)24-25-18/h8-10,12H,2-7H2,1H3,(H2,20,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated
US Patent
| Assay Description
Compounds are screened for their ability to inhibit the phosphorylation of tyrosine (TYR) residues through the use of western blotting of Jurkat cell... |
US Patent US8664219 (2014)
BindingDB Entry DOI: 10.7270/Q24748J8 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM610453

(2-Phenyl-N-(4-(2,2,2-trifluoroethoxy)pyridin-3-yl)...)Show SMILES FC(F)(F)COc1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610457

(N-(4-(4-Cyanophenyl)pyridin-3-yl)-2-phenylimidazo[...)Show SMILES O=C(Nc1cnccc1-c1ccc(cc1)C#N)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610469

((S)�N-(4-(3-Methylmorpholino)pyridin-3-yl)-2-pheny...)Show SMILES C[C@H]1COCCN1c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM610463

(BDBM610464 | BDBM610465 | N-(4-(2-Methylmorpholino...)Show SMILES CC1CN(CCO1)c1ccncc1NC(=O)c1ccnn2cc(nc12)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NC659P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
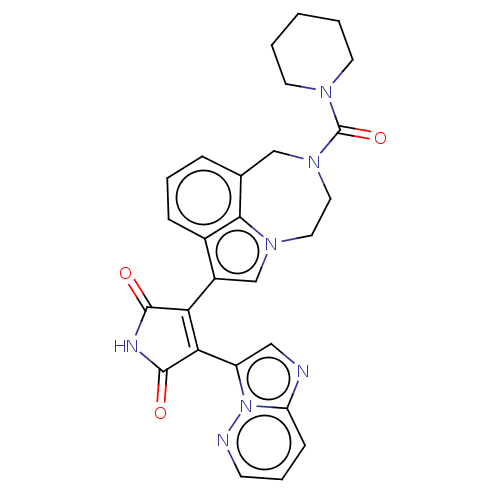
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data