

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107605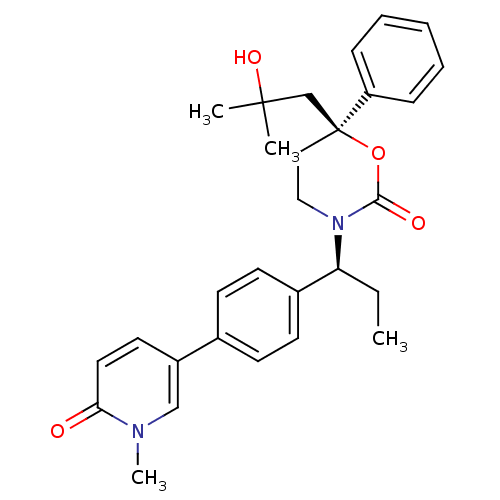 (US8575157, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107666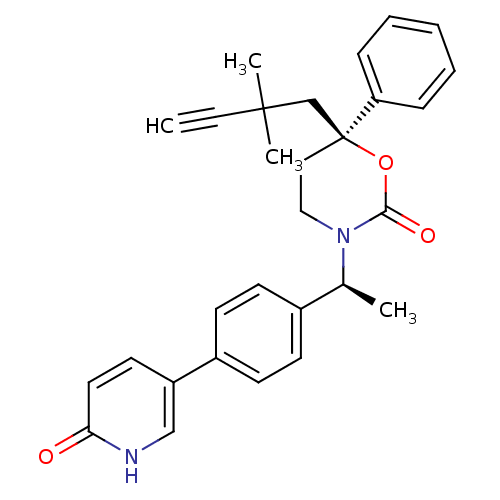 (US8575157, 230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107588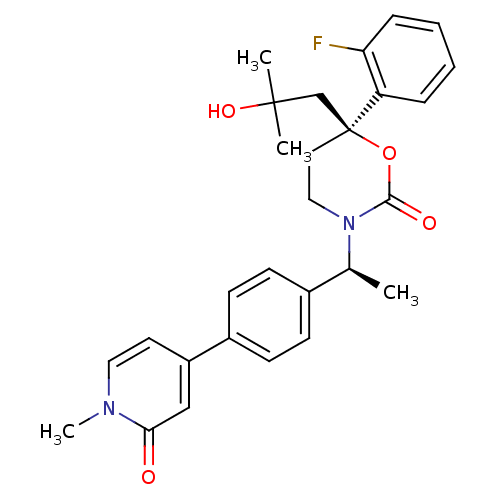 (US8575157, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107594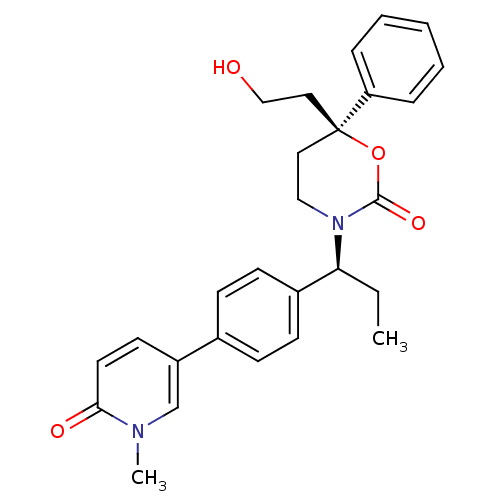 (US8575157, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107601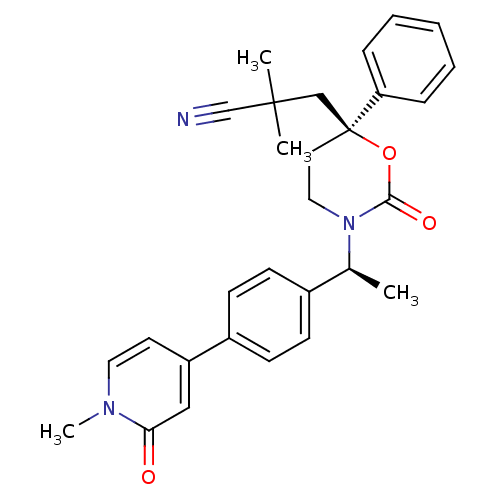 (US8575157, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107612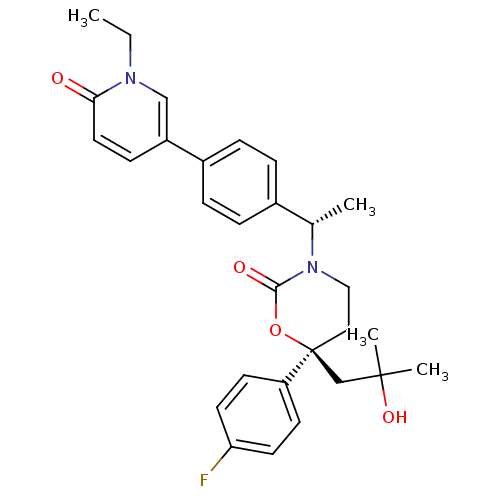 (US8575157, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107623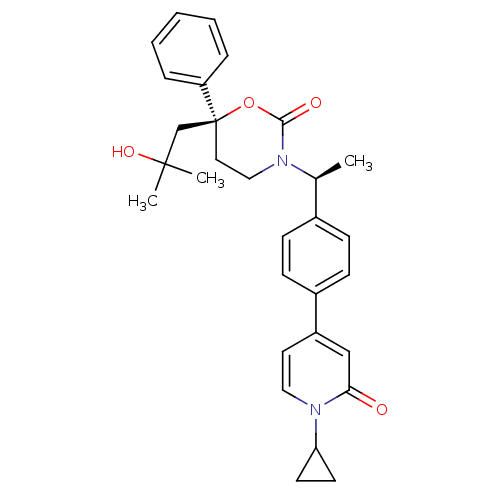 (US8575157, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107629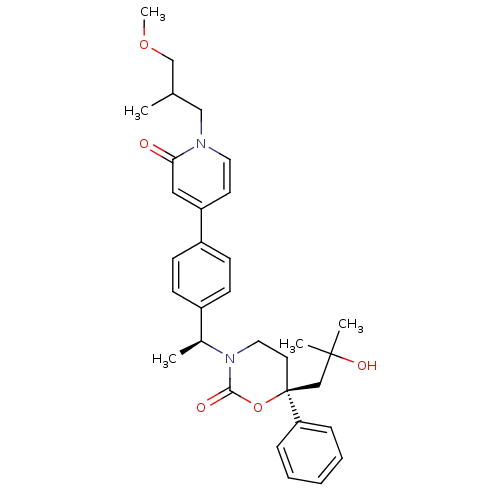 (US8575157, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107588 (US8575157, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107657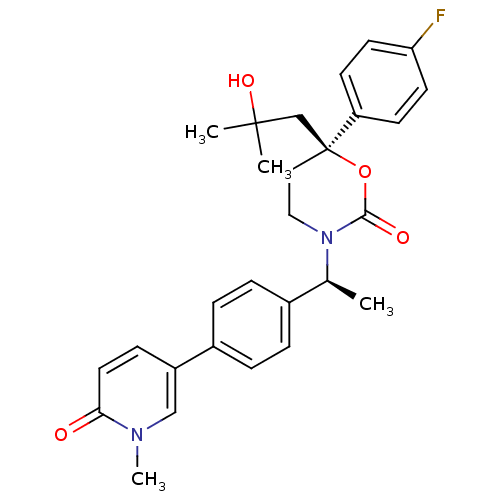 (US8575157, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107658 (US8575157, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107594 (US8575157, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107609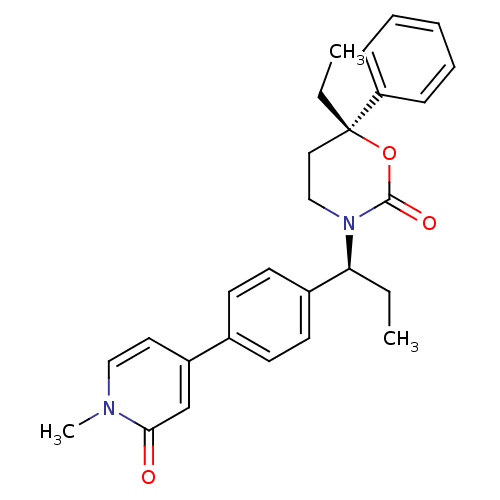 (US8575157, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107603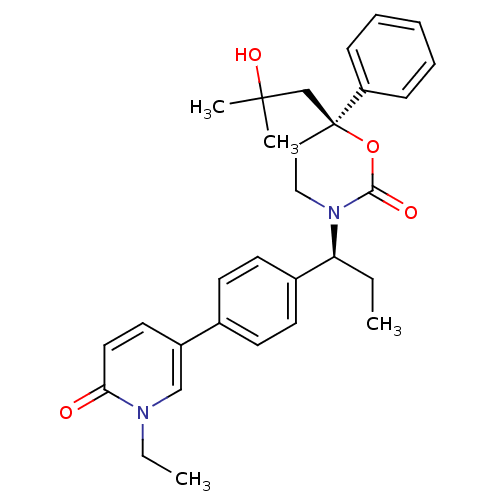 (US8575157, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107665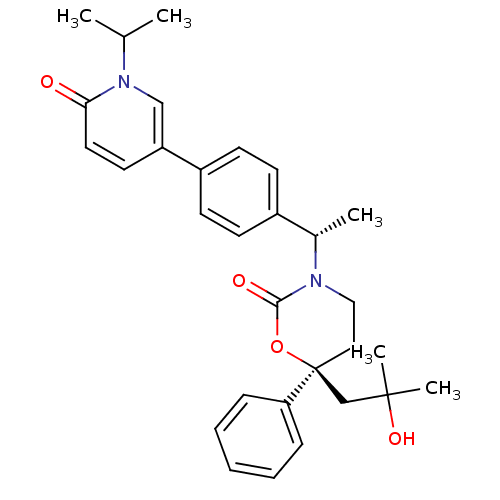 (US8575157, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107612 (US8575157, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107619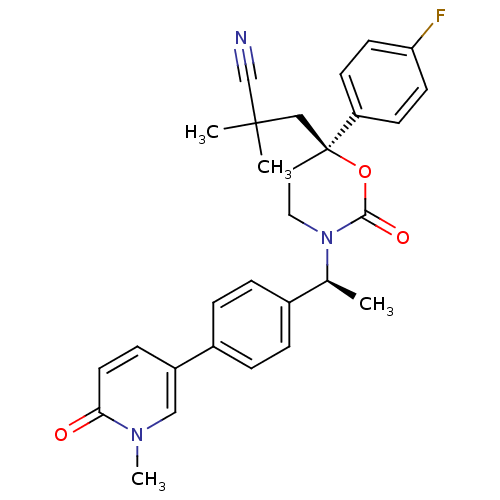 (US8575157, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107629 (US8575157, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107633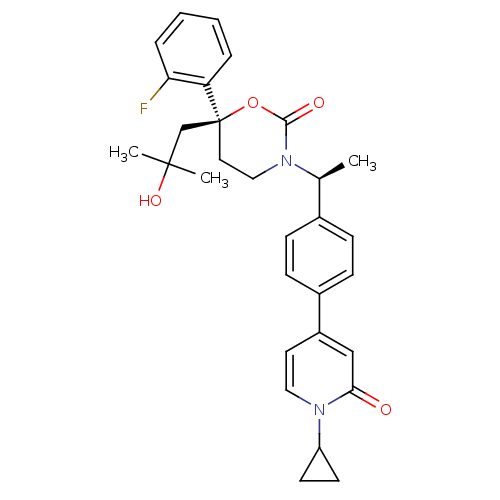 (US8575157, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107611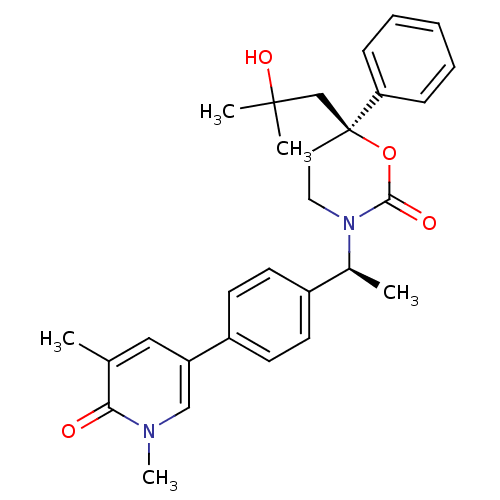 (US8575157, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107617 (US8575157, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107625 (US8575157, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107630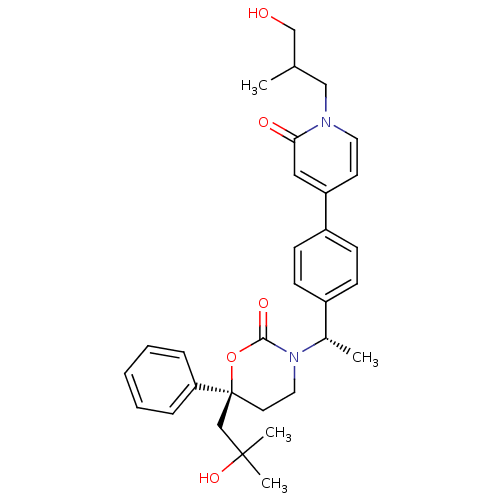 (US8575157, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107617 (US8575157, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107666 (US8575157, 230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107628 (US8575157, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107629 (US8575157, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107633 (US8575157, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107640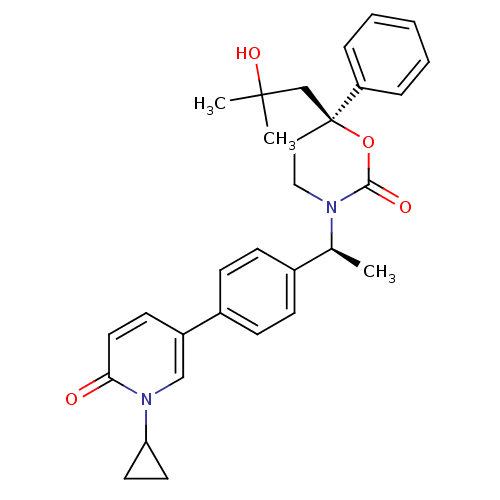 (US8575157, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107585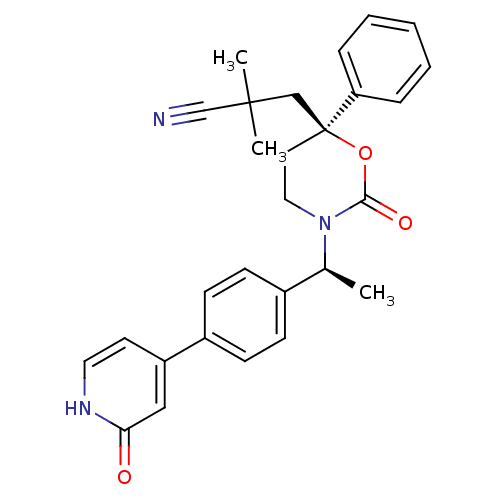 (US8575157, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107614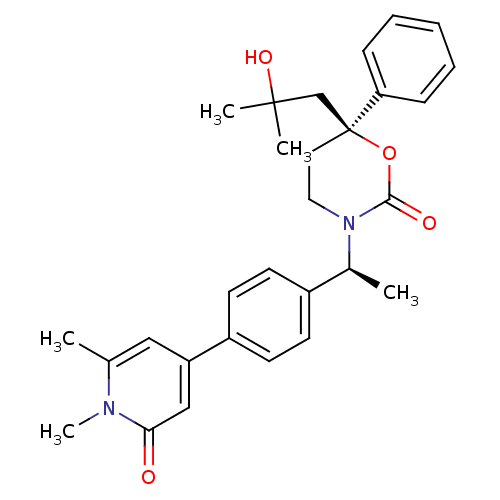 (US8575157, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107620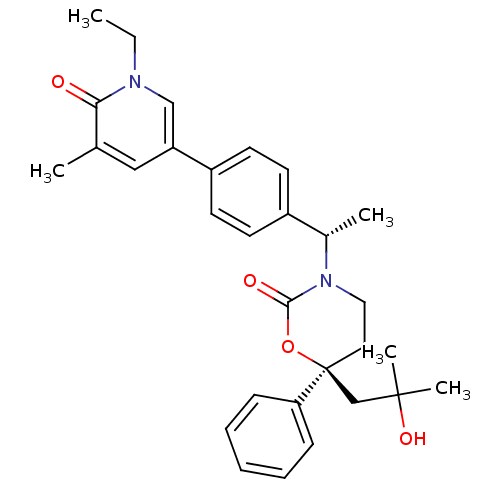 (US8575157, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107623 (US8575157, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107630 (US8575157, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107631 (US8575157, 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107585 (US8575157, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107653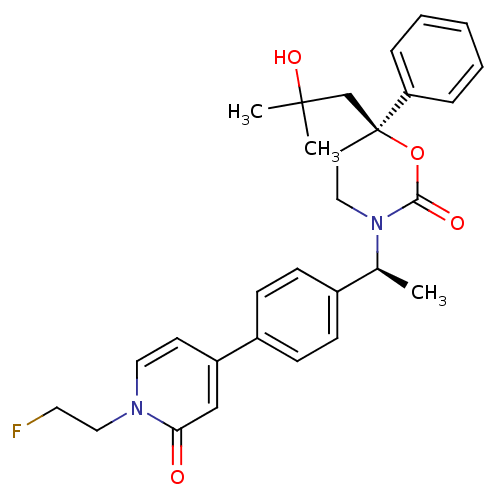 (US8575157, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107606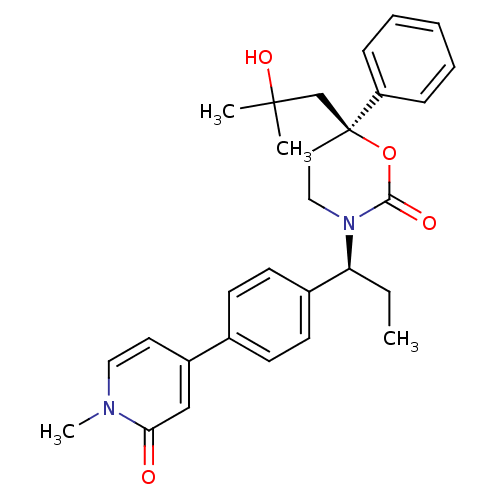 (US8575157, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107620 (US8575157, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107666 (US8575157, 230) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107624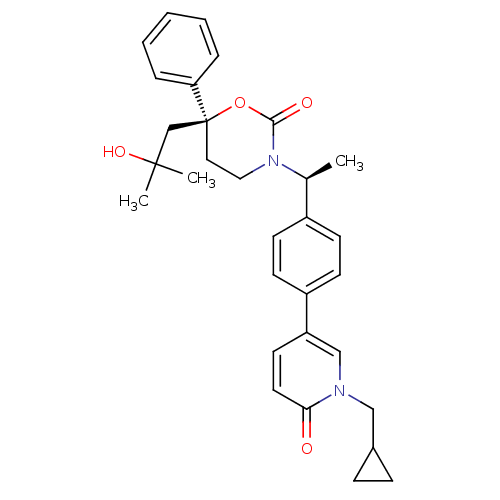 (US8575157, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107640 (US8575157, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107652 (US8575157, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107656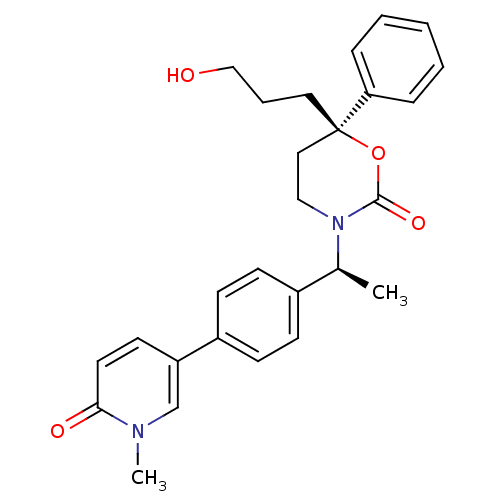 (US8575157, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107641 (US8575157, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107588 (US8575157, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107601 (US8575157, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107611 (US8575157, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107627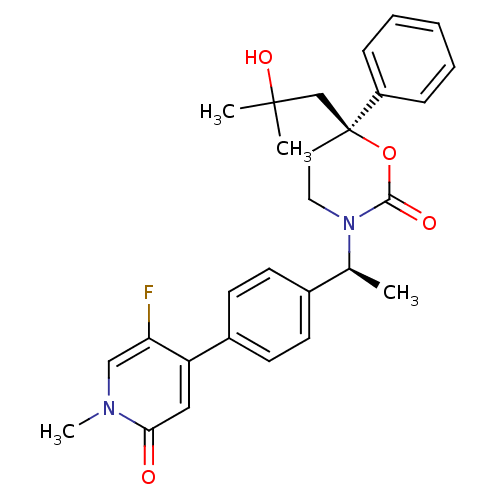 (US8575157, 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107641 (US8575157, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 341 total ) | Next | Last >> |