

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107580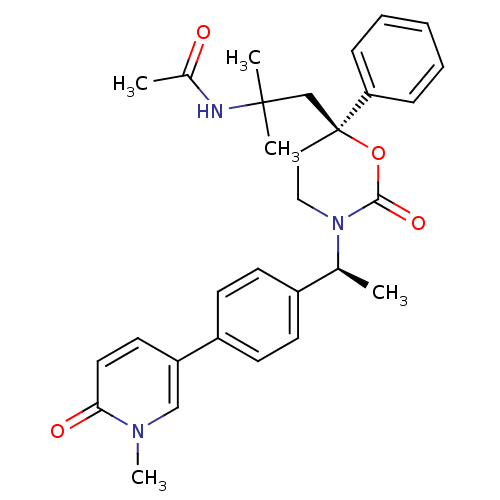 (US8575157, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107650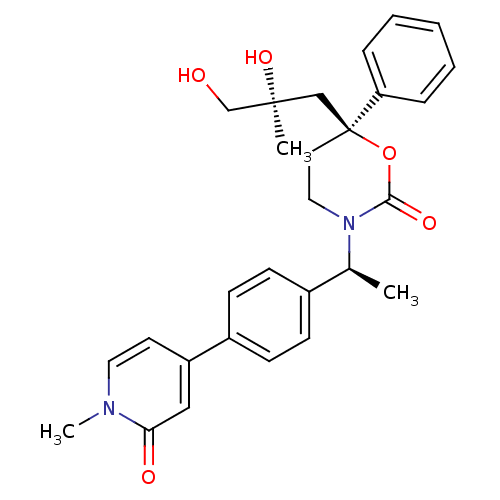 (US8575157, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107650 (US8575157, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107651 (US8575157, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107587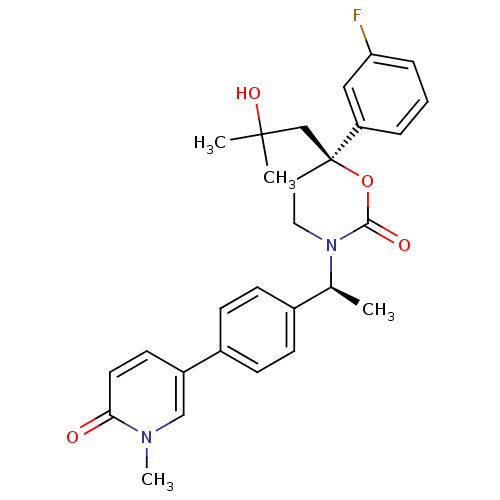 (US8575157, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107588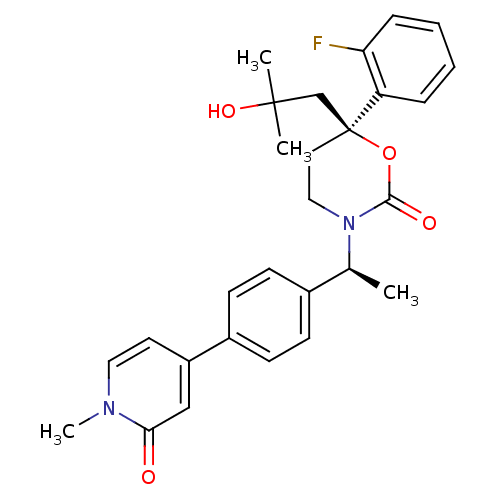 (US8575157, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107661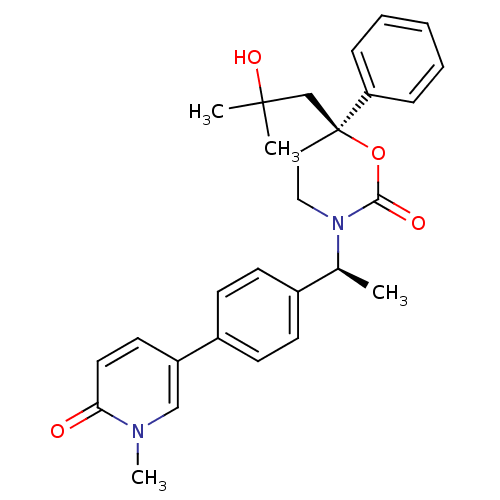 (US8575157, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107598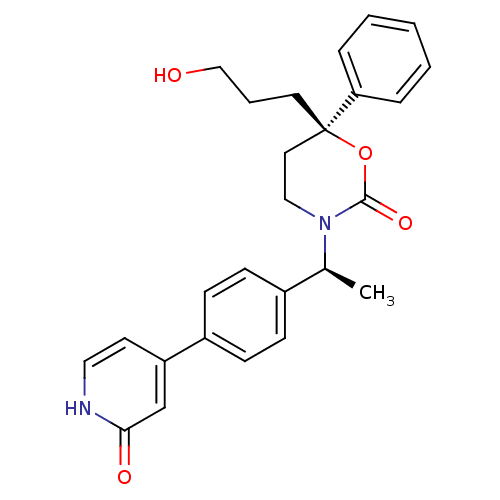 (US8575157, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107604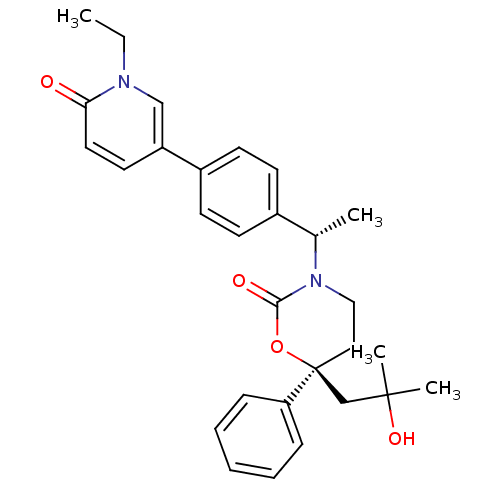 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107605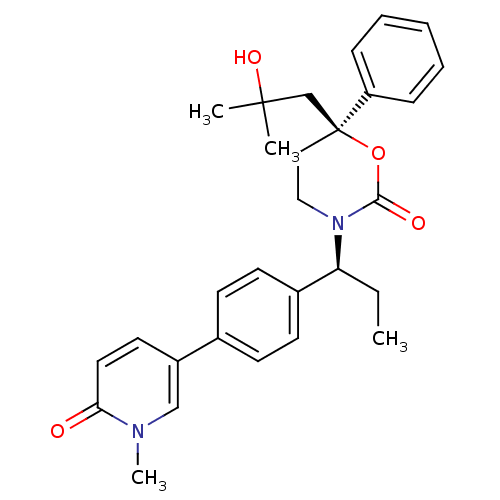 (US8575157, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107609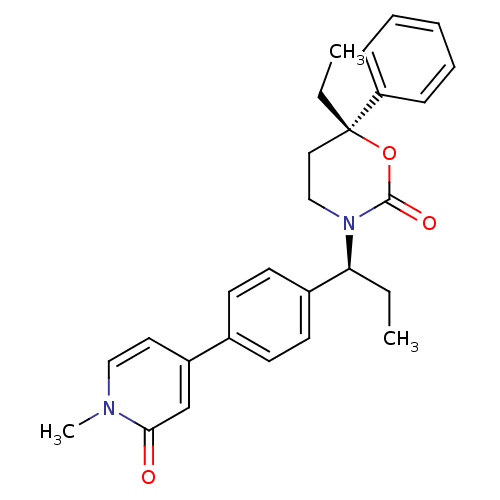 (US8575157, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107621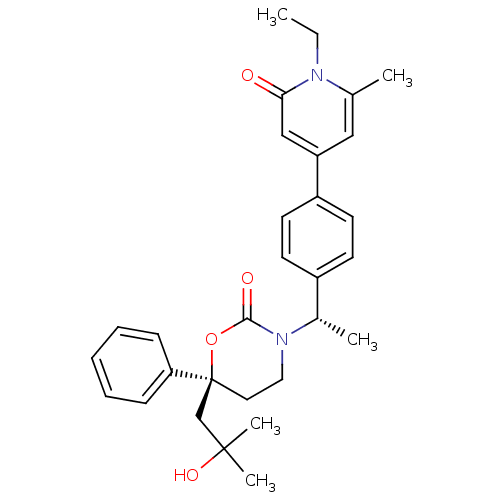 (US8575157, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107668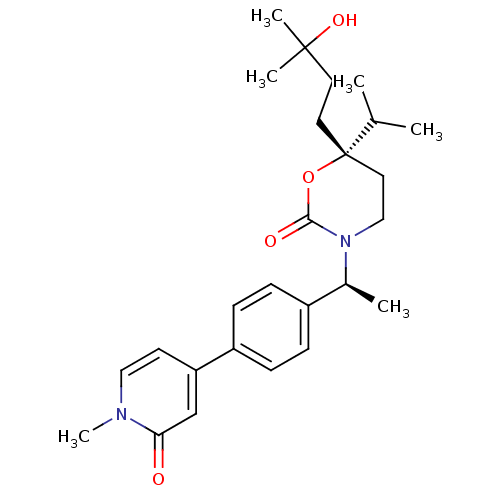 (US8575157, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107639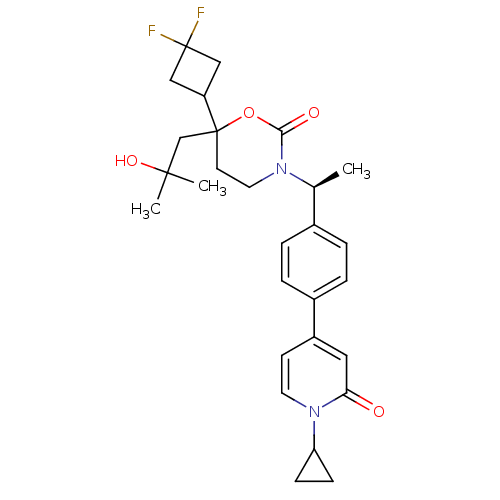 (US8575157, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50353392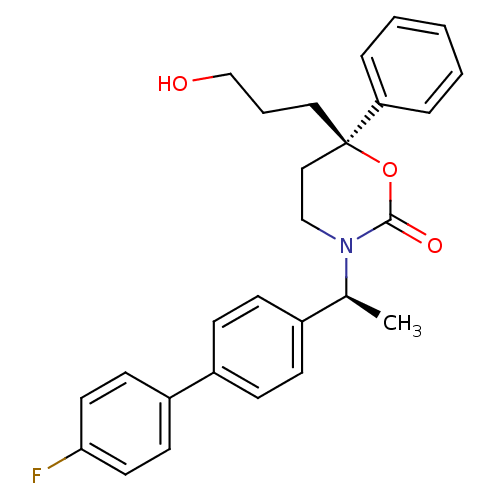 (CHEMBL1829761 | US8575157, 197 | US8592410, Compar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107582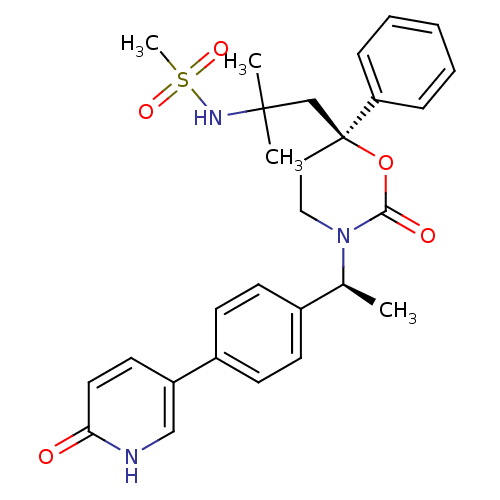 (US8575157, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107583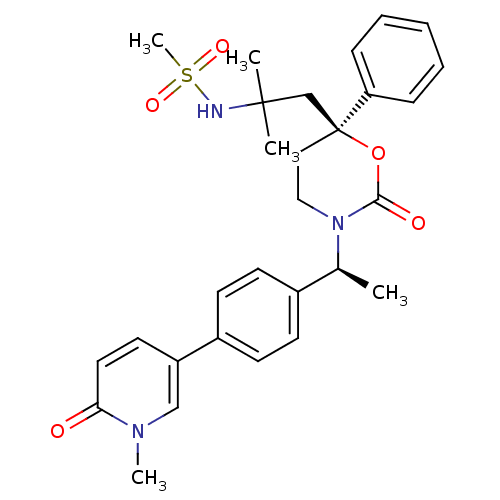 (US8575157, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107585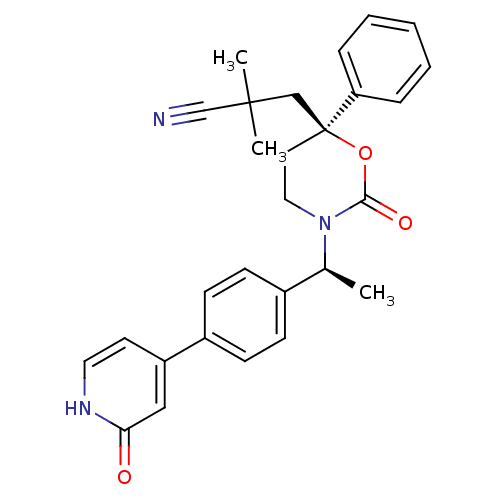 (US8575157, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107649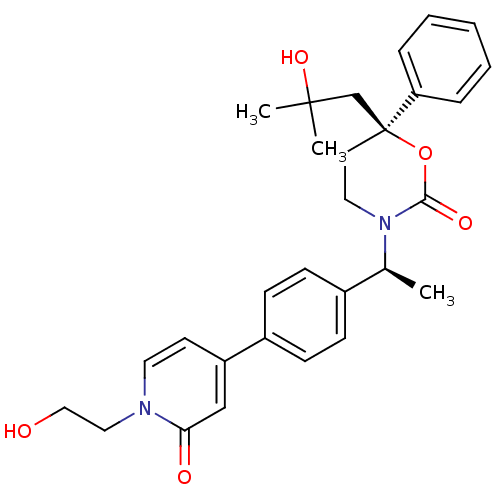 (US8575157, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107651 (US8575157, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107655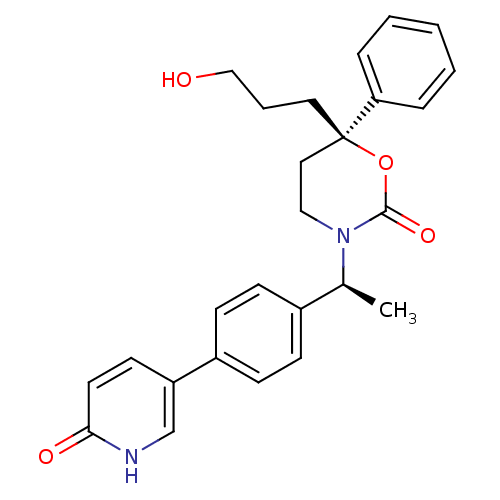 (US8575157, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107658 (US8575157, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107595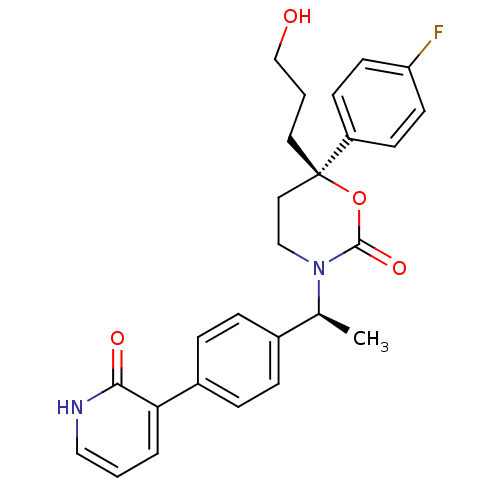 (US8575157, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107597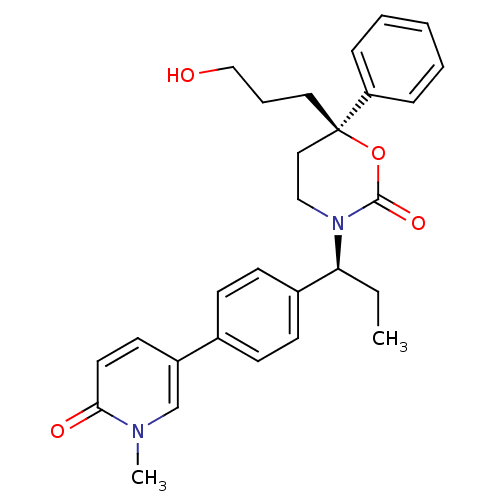 (US8575157, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107601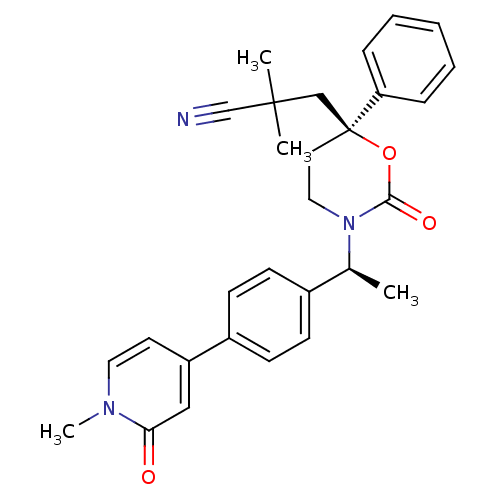 (US8575157, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107610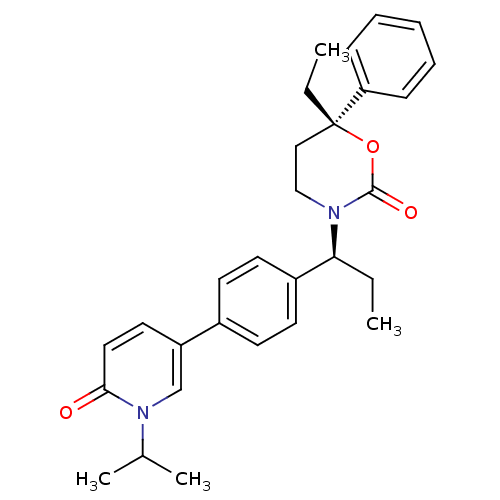 (US8575157, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107481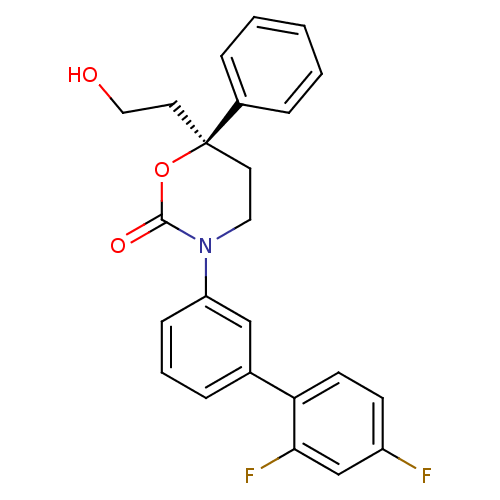 (US8575157, 189 | US8592410, Comparator 1 | US85981...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107483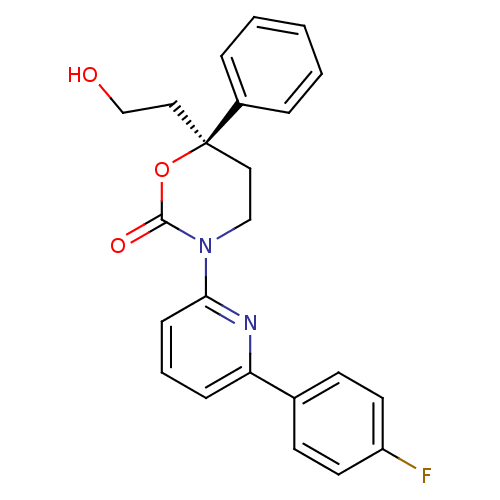 (US8575157, 192 | US8592410, Comparator 4 | US85981...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107641 (US8575157, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107585 (US8575157, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107652 (US8575157, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107600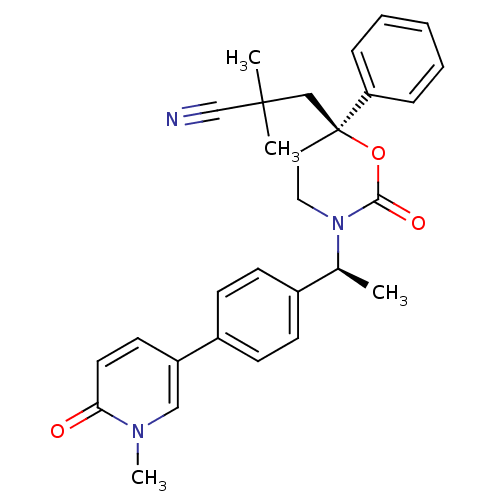 (US8575157, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107615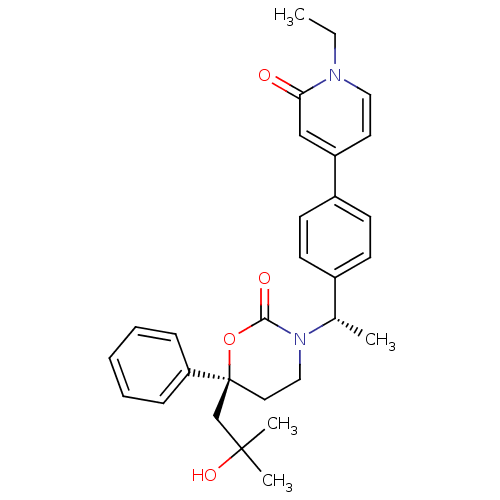 (US8575157, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107617 (US8575157, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107619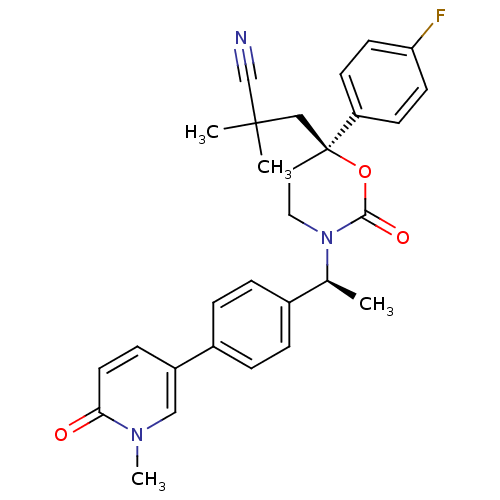 (US8575157, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107626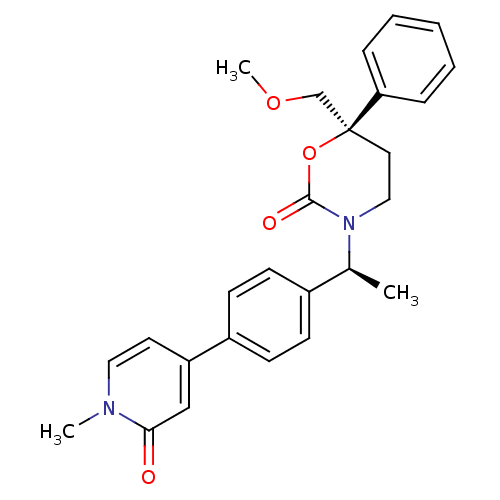 (US8575157, 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107627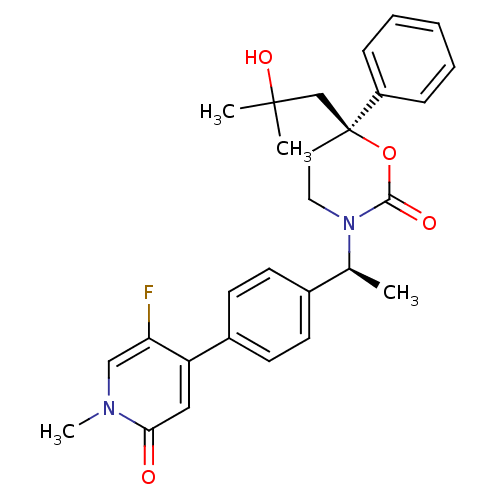 (US8575157, 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107661 (US8575157, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107594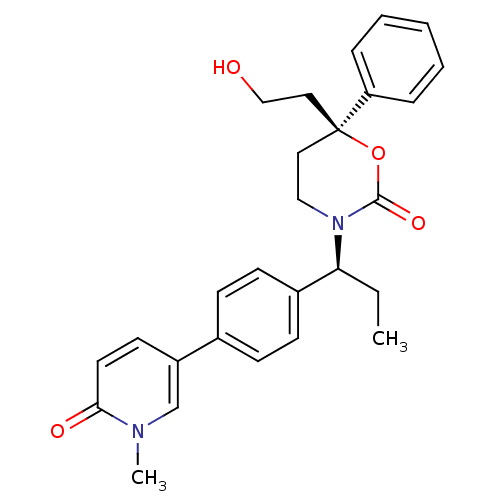 (US8575157, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107614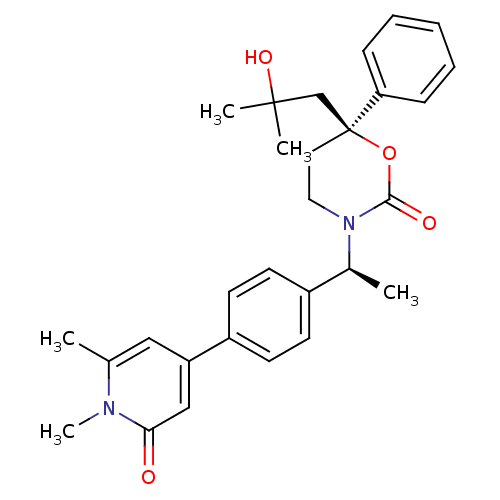 (US8575157, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107623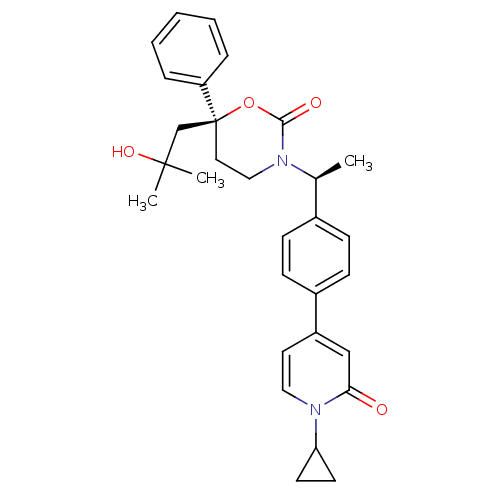 (US8575157, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107624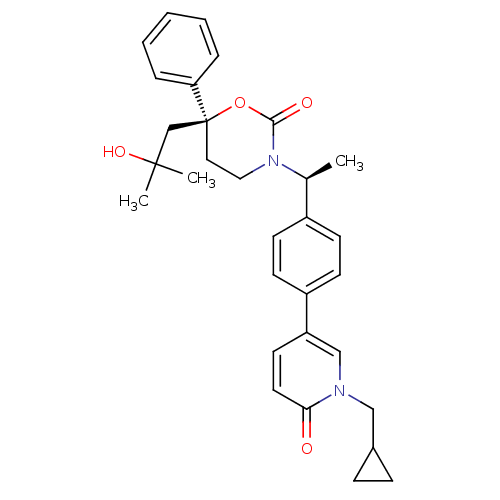 (US8575157, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107641 (US8575157, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107652 (US8575157, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107665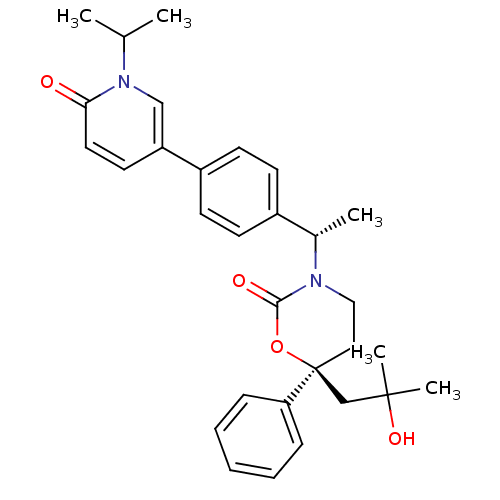 (US8575157, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107612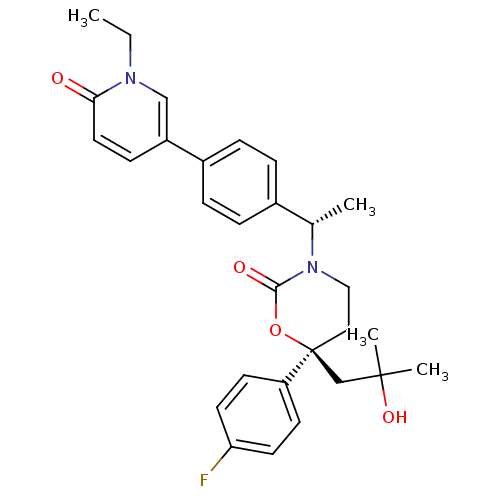 (US8575157, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107624 (US8575157, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107625 (US8575157, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107627 (US8575157, 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 341 total ) | Next | Last >> |