

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364687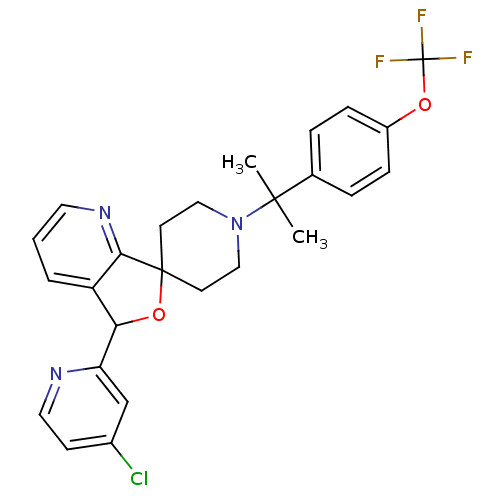 (CHEMBL1951476 | US8785634, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364676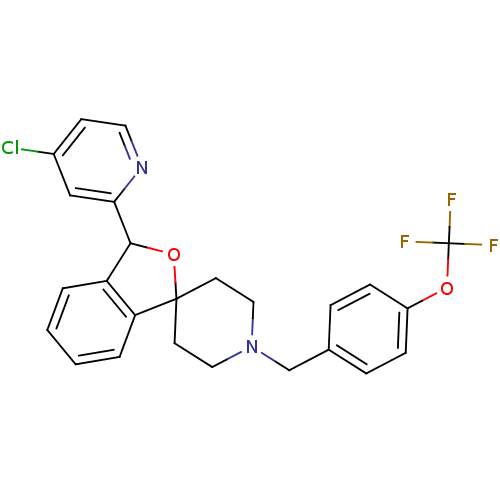 (CHEMBL1951465 | US8785634, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364679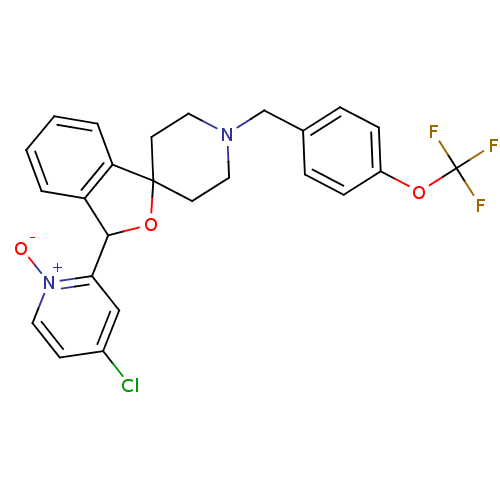 (CHEMBL1951468 | US8785634, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364682 (CHEMBL1951471 | US8785634, 82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364672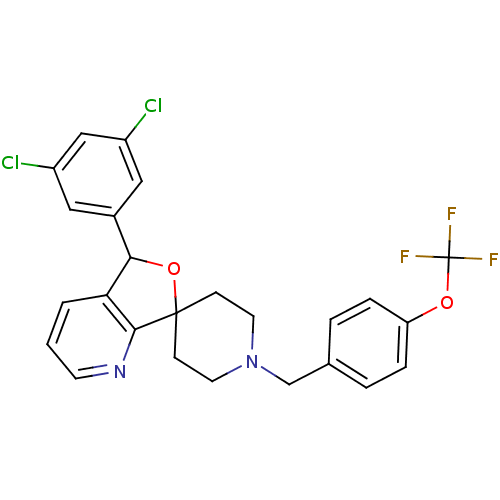 (CHEMBL1951461 | US8785634, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM126716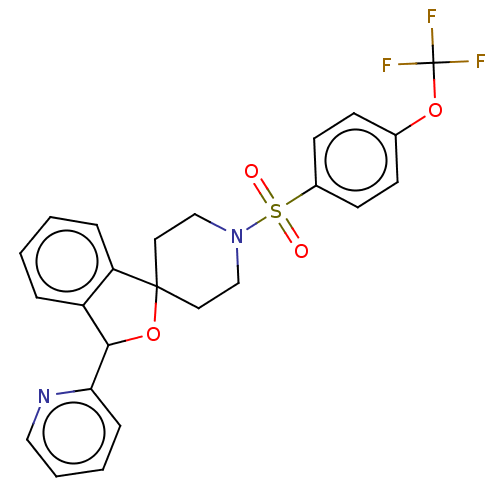 (US8785634, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM126715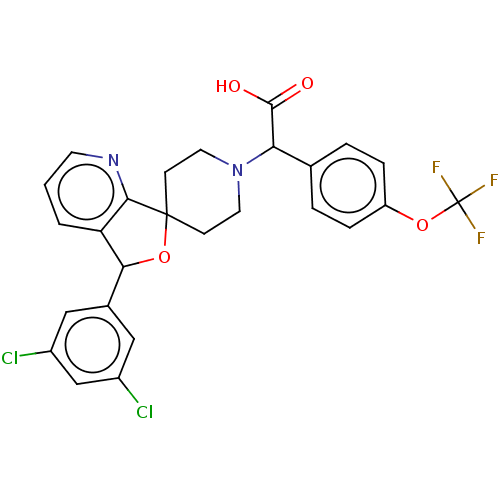 (US8785634, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM126713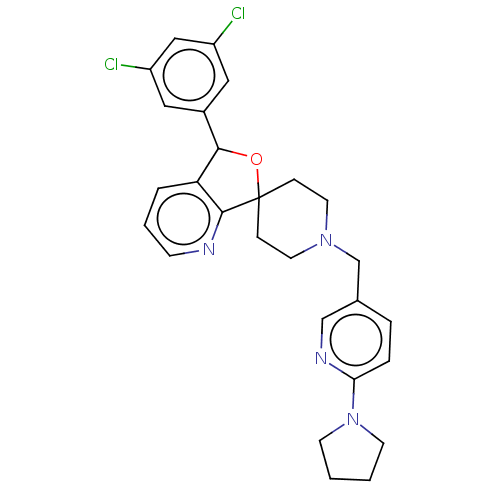 (US8785634, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM126714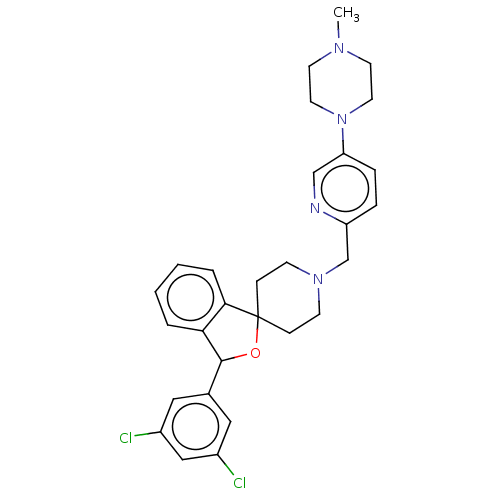 (US8785634, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||