

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138989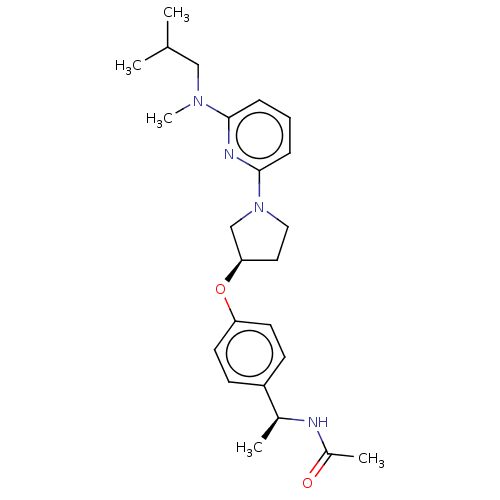 (US8877741, 10.36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 399 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138992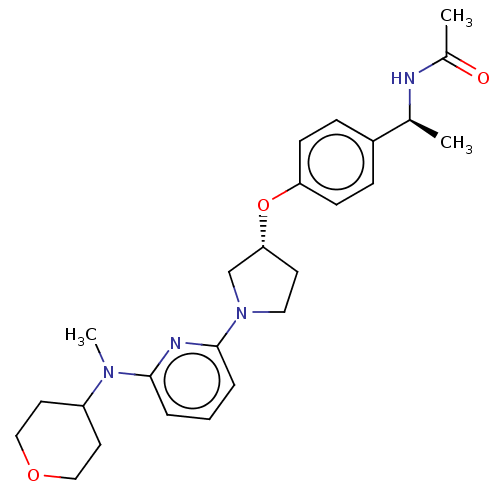 (US8877741, 10.39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139002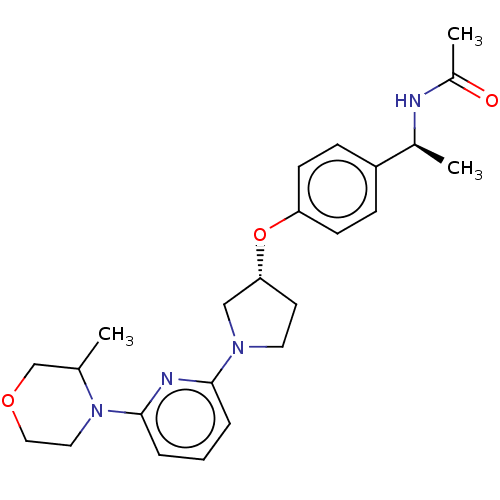 (US8877741, 10.49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 785 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139003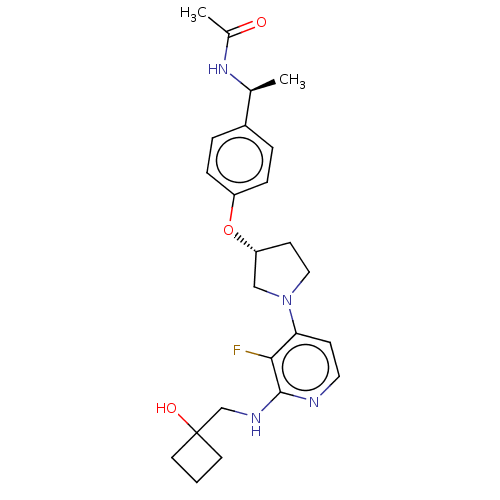 (US8877741, 10.50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139009 (US8877741, 11.1 | US8877741, 859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139013 (US8877741, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 888 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138527 (US8877741, 1.5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138536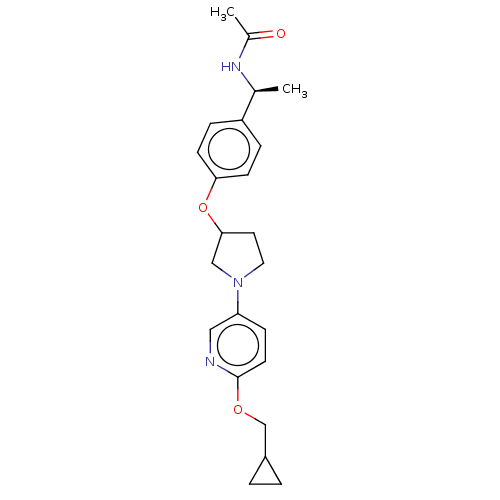 (US8877741, 1.14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 394 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138547 (US8877741, 1.25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 228 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138560 (US8877741, 1.38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138566 (US8877741, 1.44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 256 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138574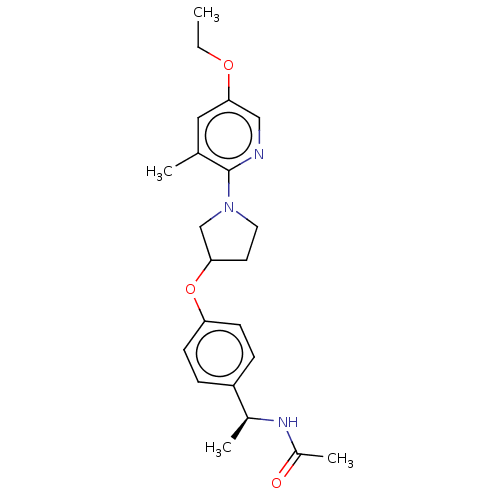 (US8877741, 1.52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 474 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138579 (US8877741, 1.57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 678 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138585 (US8877741, 1.63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138589 (US8877741, 1.67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 463 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138591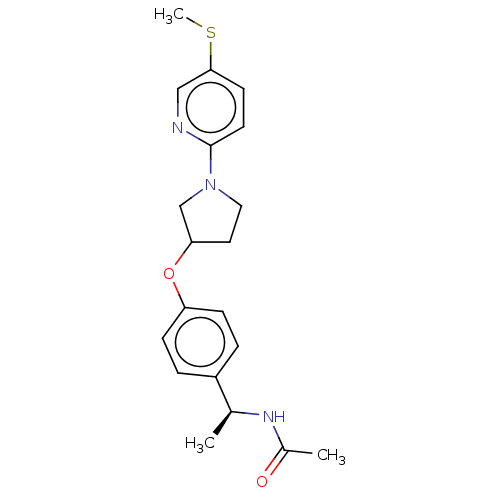 (US8877741, 1.69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 387 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138607 (US8877741, 1.85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138617 (US8877741, 1.95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 82 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138622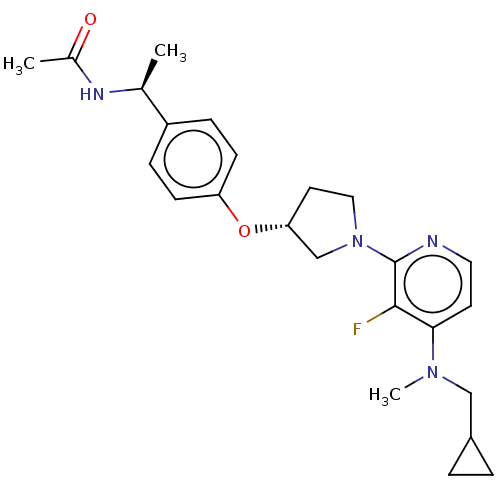 (US8877741, 1.100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 385 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138624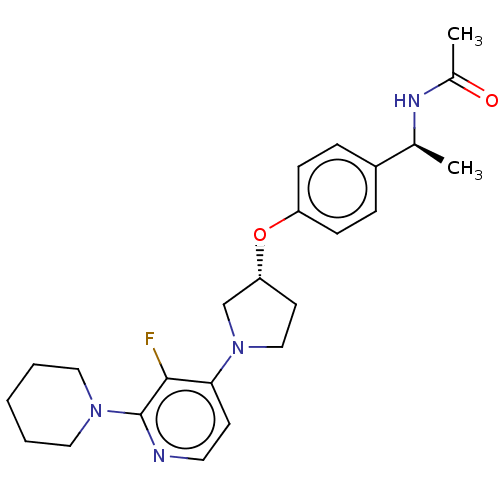 (US8877741, 1.102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138630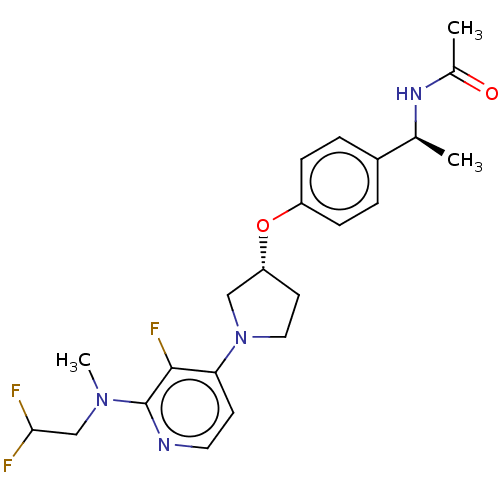 (US8877741, 1.108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138631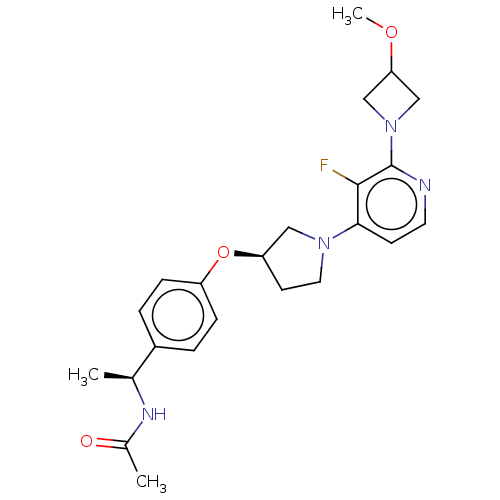 (US8877741, 1.109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 155 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138635 (US8877741, 1.113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138646 (US8877741, 1.124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138661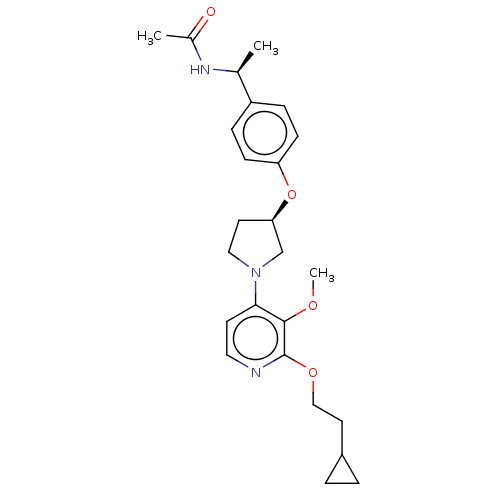 (US8877741, 1.139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138664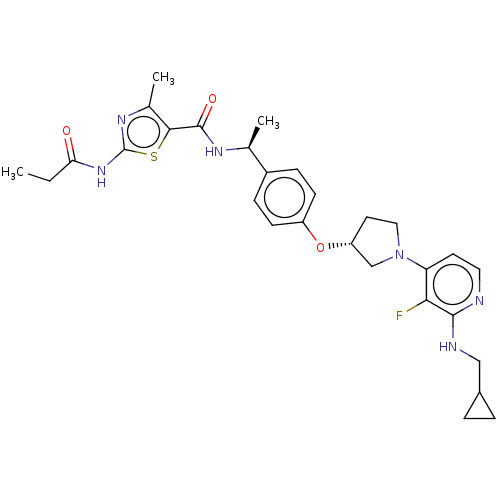 (US8877741, 1.142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138683 (US8877741, 2.9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 873 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138684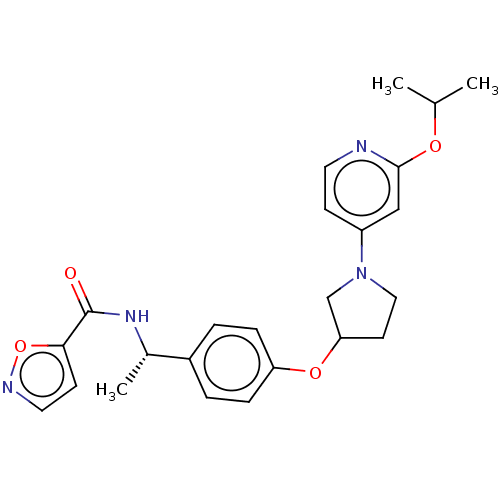 (US8877741, 2.10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 670 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138685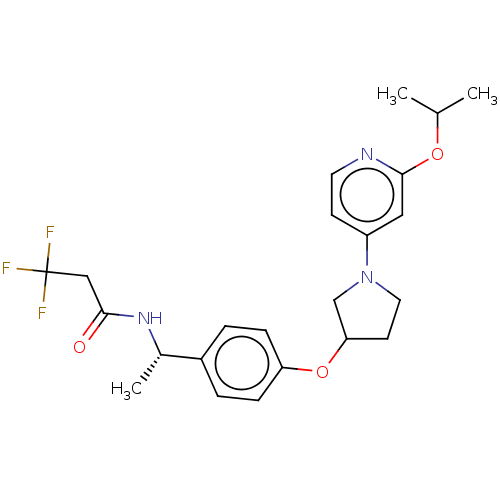 (US8877741, 2.11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138694 (US8877741, 2.20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138710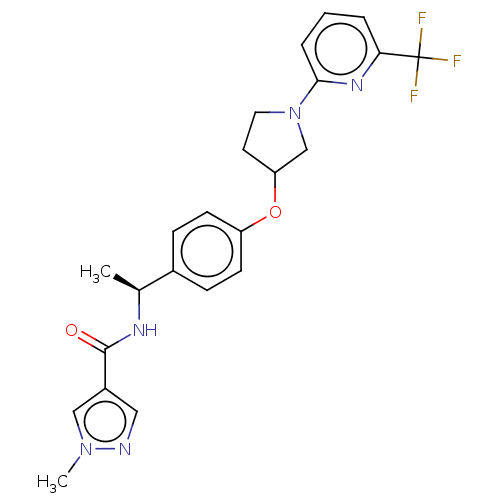 (US8877741, 2.36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138712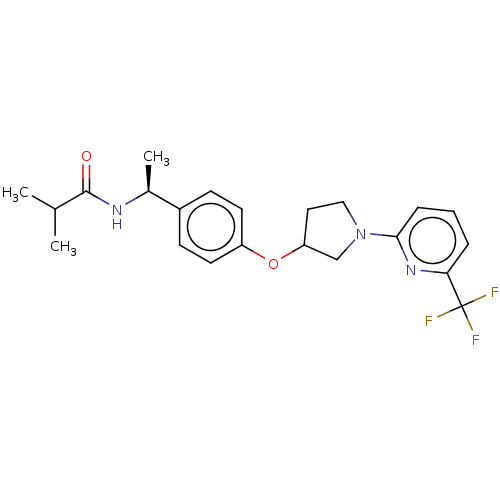 (US8877741, 2.38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 869 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138717 (US8877741, 2.43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138723 (US8877741, 2.49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138739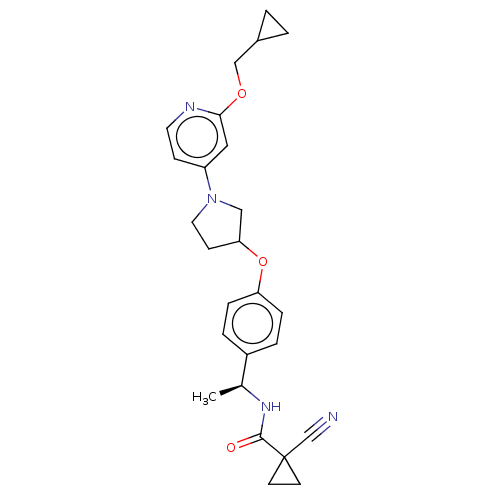 (US8877741, 2.65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138745 (US8877741, 2.71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138746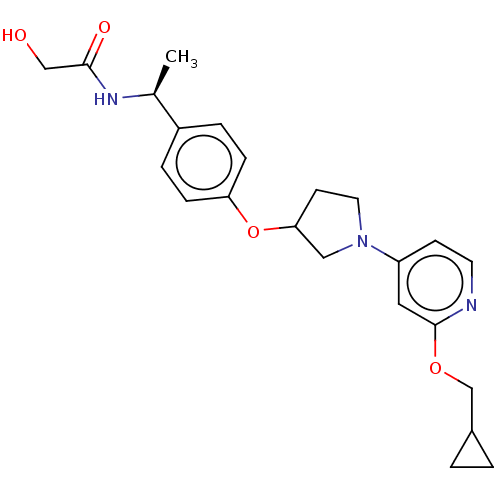 (US8877741, 2.72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138747 (US8877741, 2.73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138764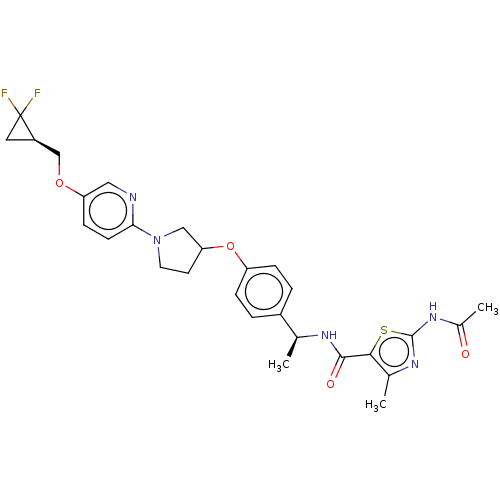 (US8877741, 2.90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138765 (US8877741, 2.91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138770 (US8877741, 2.96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138776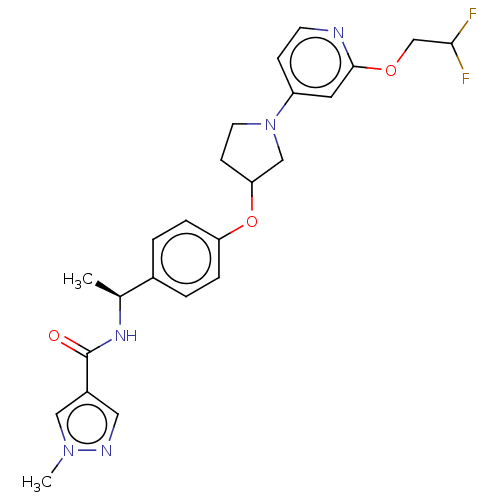 (US8877741, 2.102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138780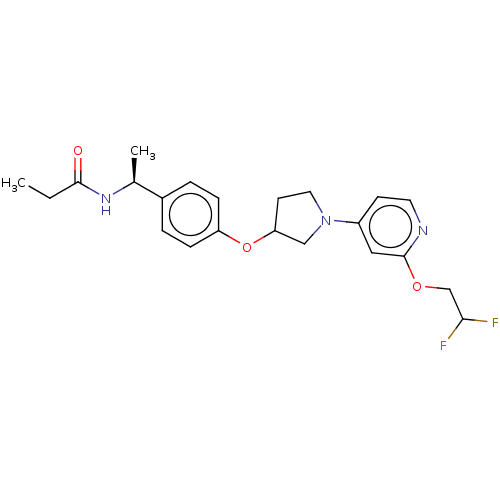 (US8877741, 2.106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138797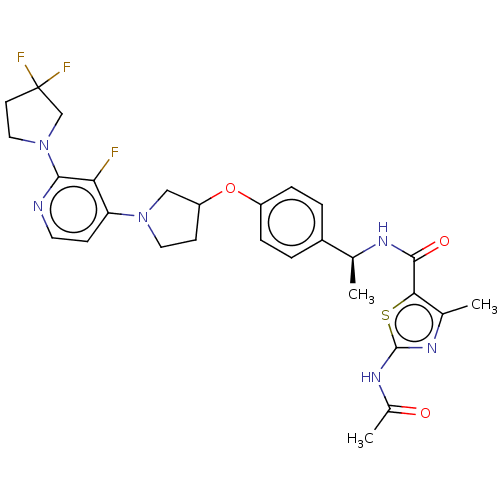 (US8877741, 2.123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138801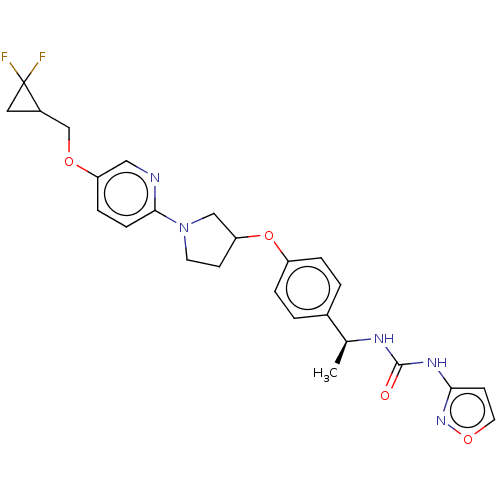 (US8877741, 3.1 | US8877741, 651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138814 (US8877741, 3.13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 497 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138823 (US8877741, 3.22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138829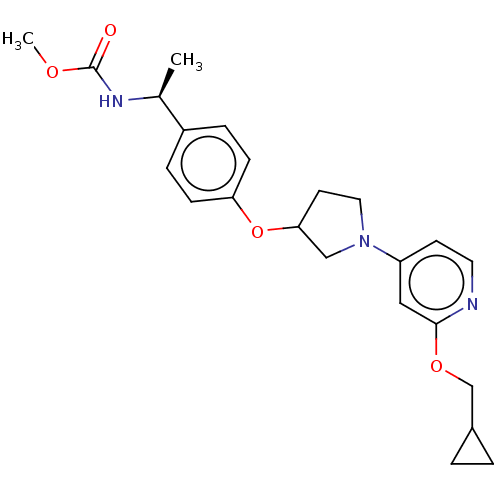 (US8877741, 4.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138833 (US8877741, 4.8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138835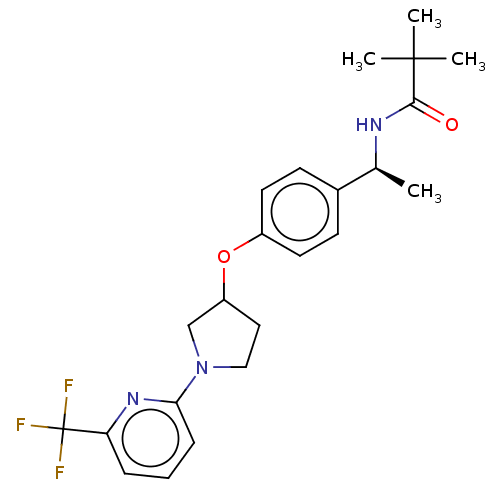 (US8877741, 5.1 | US8877741, 685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 649 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 485 total ) | Next | Last >> |