

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138997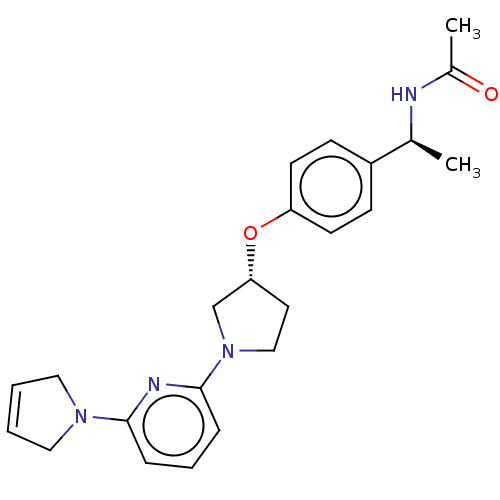 (US8877741, 10.44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 665 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139001 (US8877741, 10.48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 256 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139011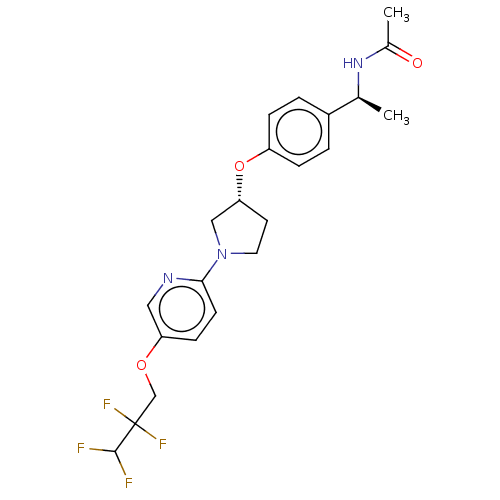 (US8877741, 11.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 870 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138525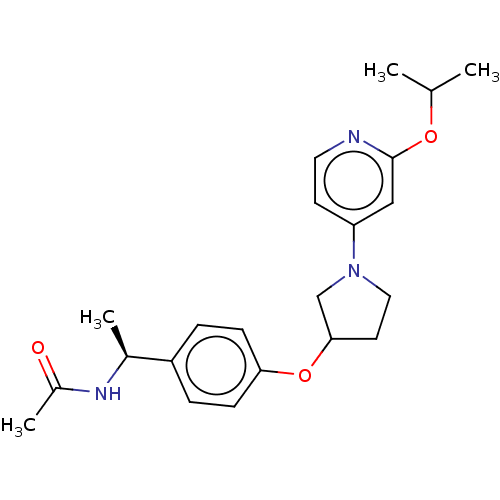 (US8877741, 1.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 134 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138535 (US8877741, 1.13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 345 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138537 (US8877741, 1.15 | US8877741, 1.35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138543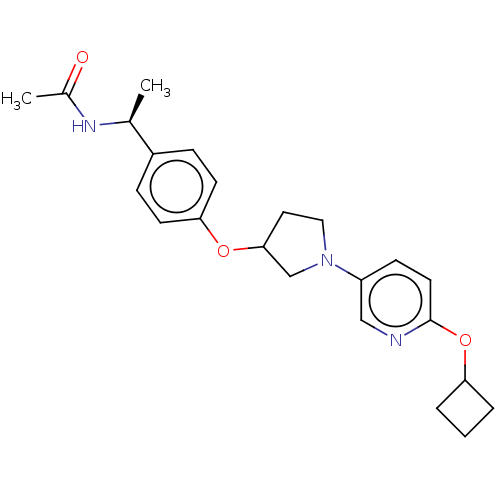 (US8877741, 1.21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 335 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138579 (US8877741, 1.57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 678 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138586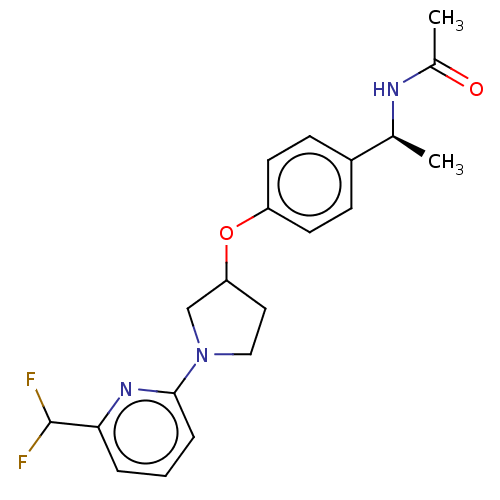 (US8877741, 1.64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138609 (US8877741, 1.87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 677 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138610 (US8877741, 1.88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 423 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138616 (US8877741, 1.94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138619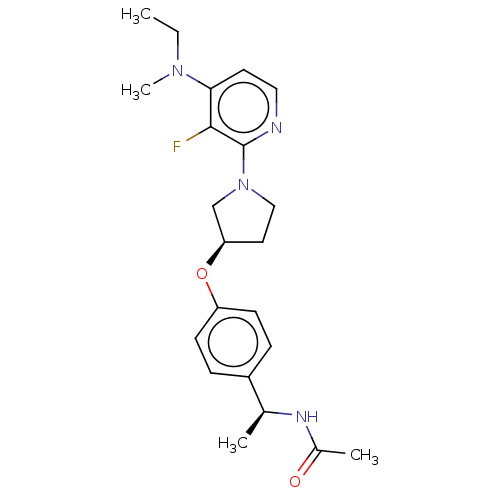 (US8877741, 1.97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 970 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138633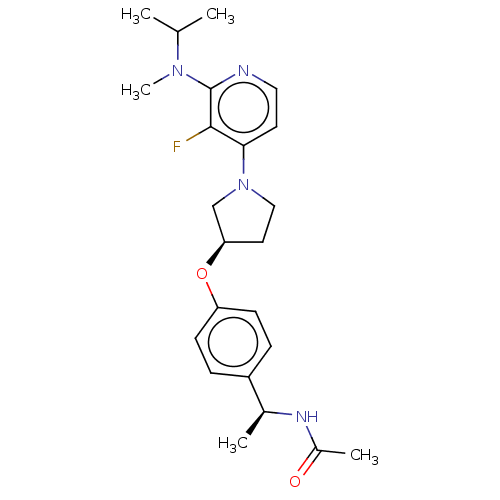 (US8877741, 1.111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138635 (US8877741, 1.113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138647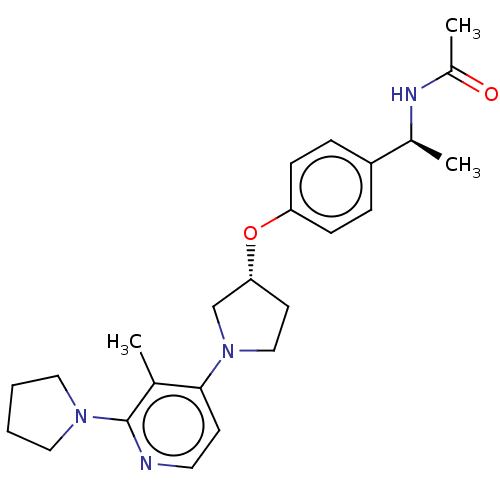 (US8877741, 1.125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 402 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138650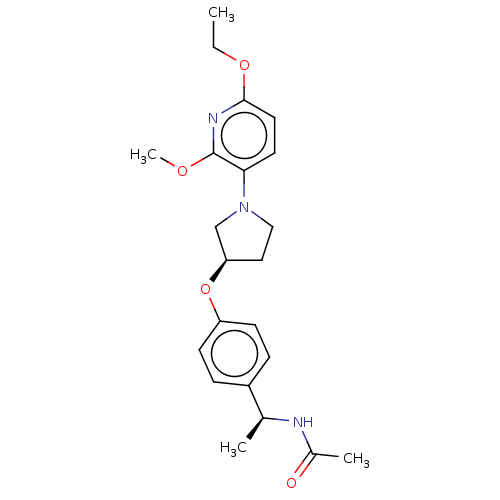 (US8877741, 1.128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138654 (US8877741, 1.132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138662 (US8877741, 1.140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138663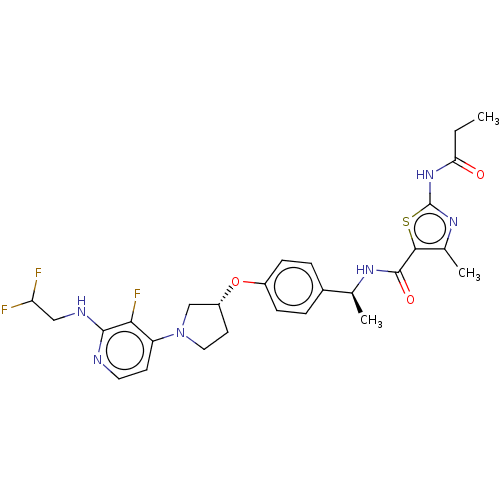 (US8877741, 1.141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 421 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138665 (US8877741, 1.143) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 116 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138666 (US8877741, 1.144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138670 (US8877741, 1.148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138691 (US8877741, 2.17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 607 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138699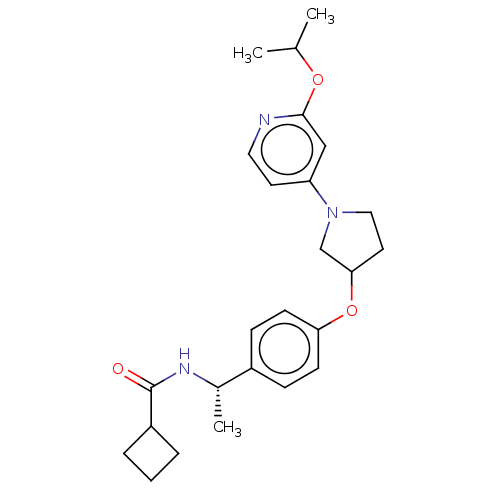 (US8877741, 2.25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 564 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138711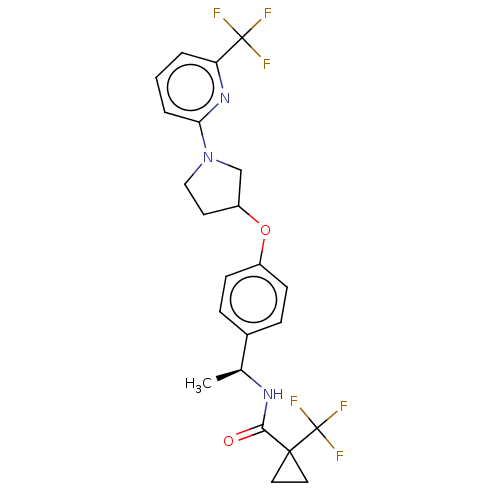 (US8877741, 2.37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 247 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138731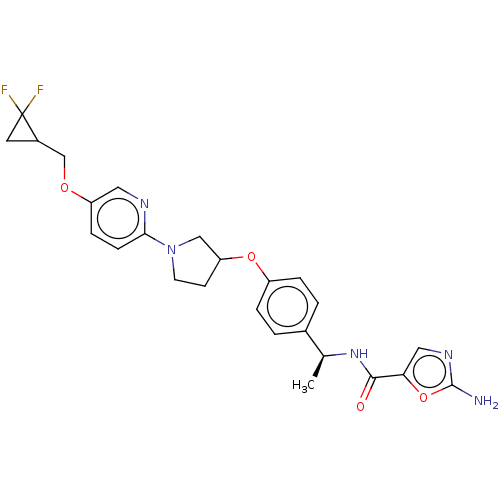 (US8877741, 2.57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 255 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138739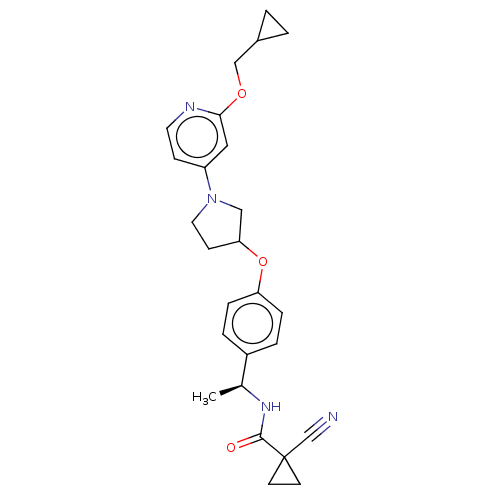 (US8877741, 2.65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138742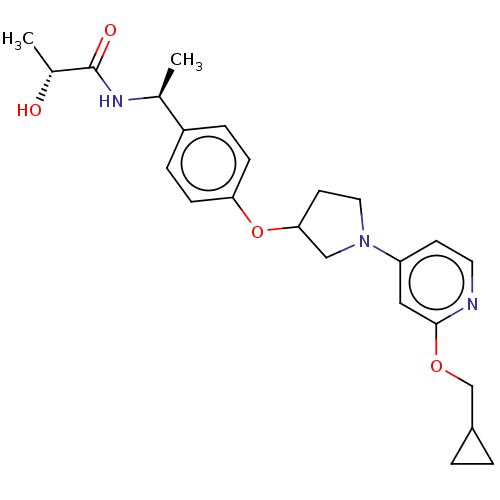 (US8877741, 2.68 | US8877741, 2.78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 265 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138743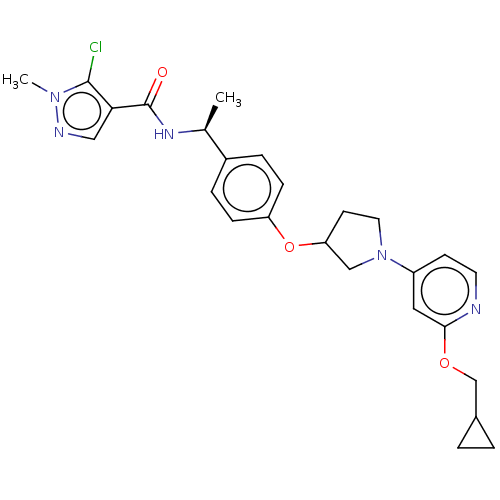 (US8877741, 2.69 | US8877741, 2.76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138764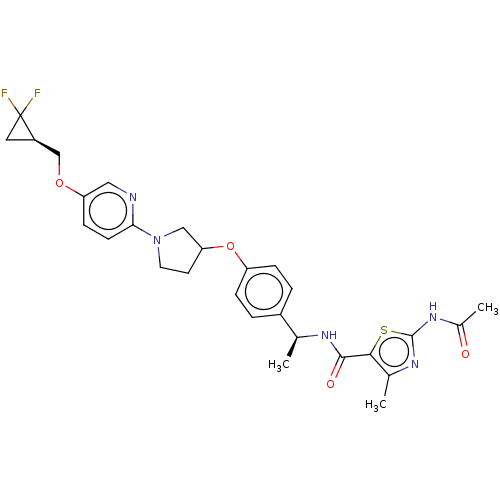 (US8877741, 2.90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138766 (US8877741, 2.92) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138767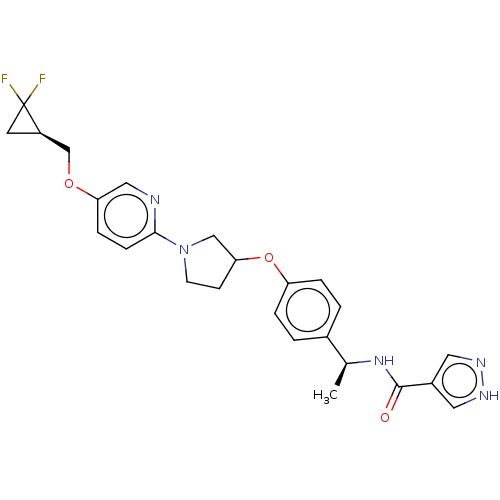 (US8877741, 2.93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138784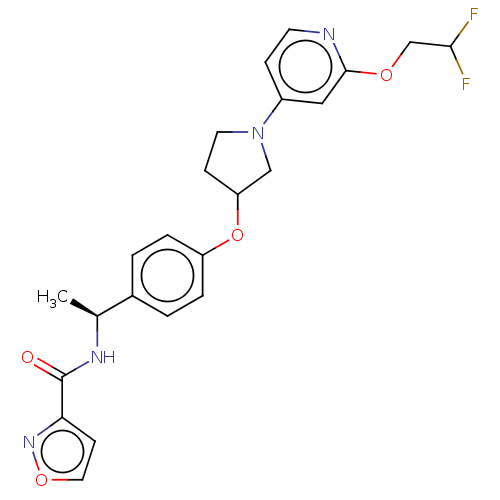 (US8877741, 2.110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138788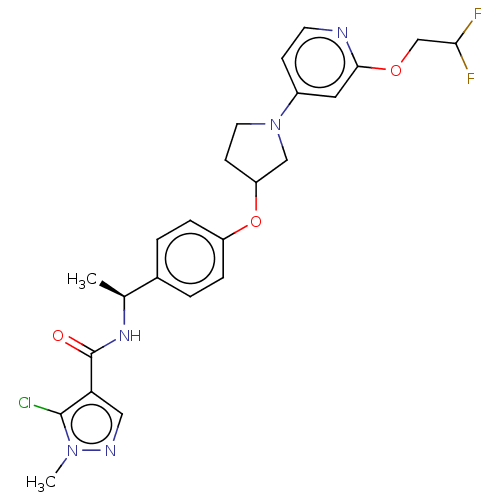 (US8877741, 2.114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 611 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138796 (US8877741, 2.122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138800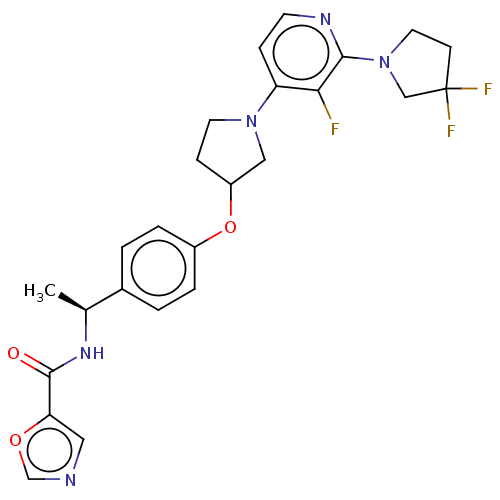 (US8877741, 2.126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138804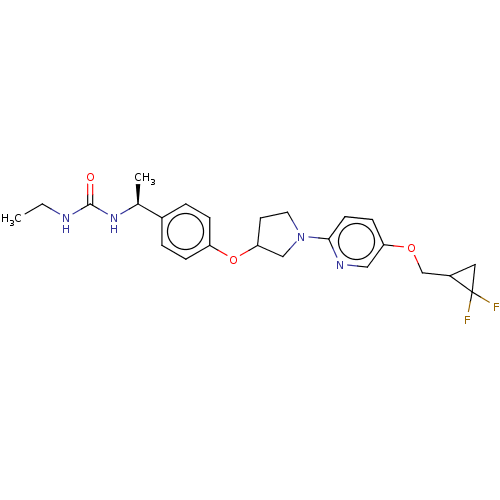 (US8877741, 3.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138809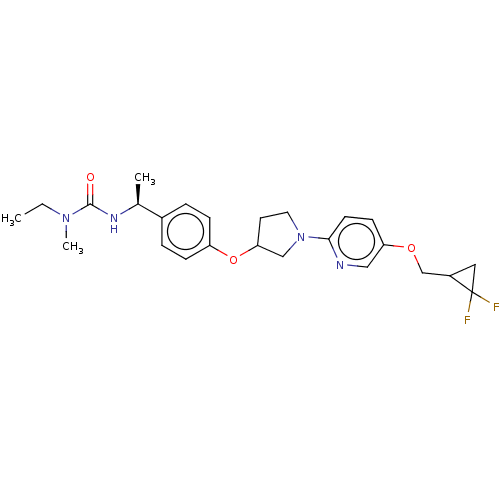 (US8877741, 3.8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138814 (US8877741, 3.13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 497 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138822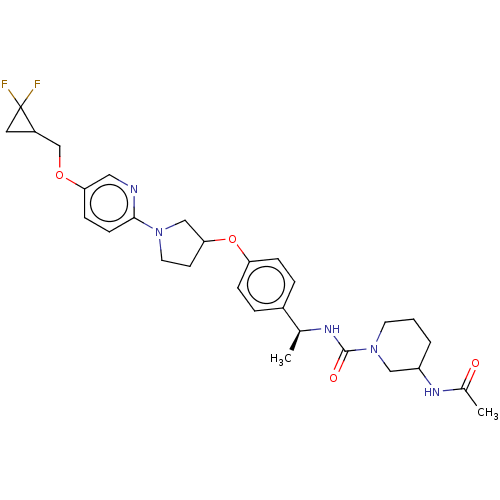 (US8877741, 3.21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 363 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138823 (US8877741, 3.22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138838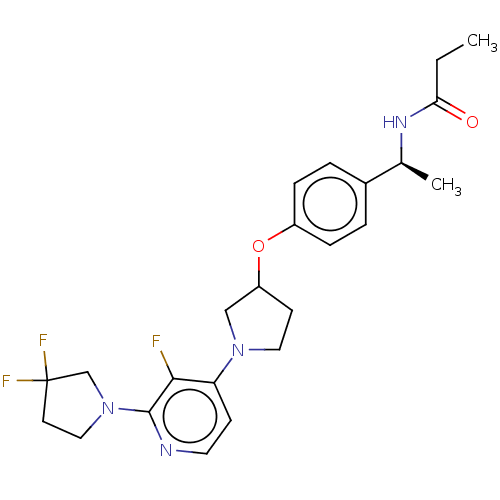 (US8877741, 5.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138855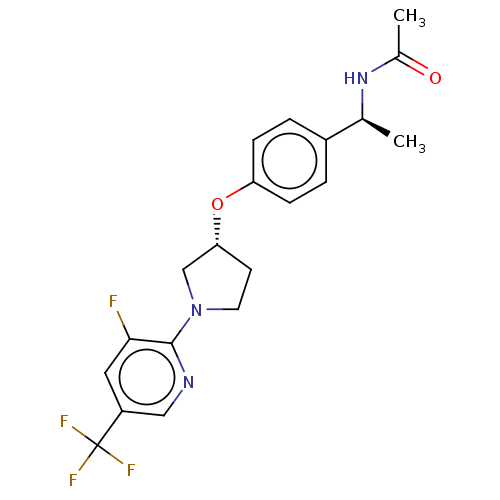 (US8877741, 8.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 755 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138863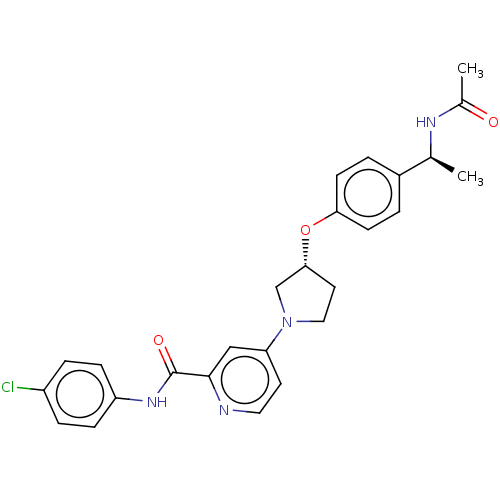 (US8877741, 8.11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138874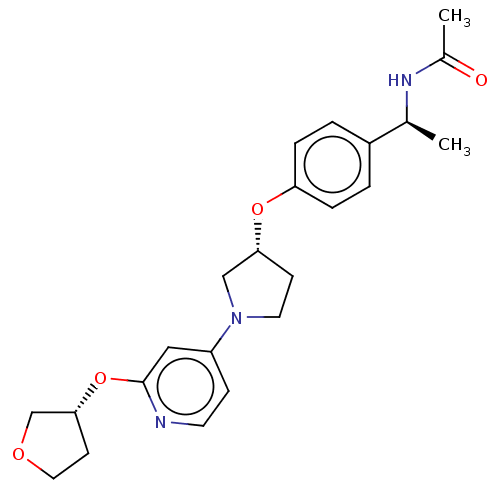 (US8877741, 9.10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 184 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138910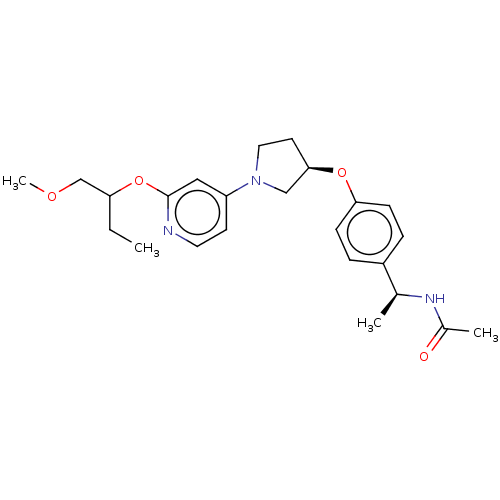 (US8877741, 9.46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 544 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138915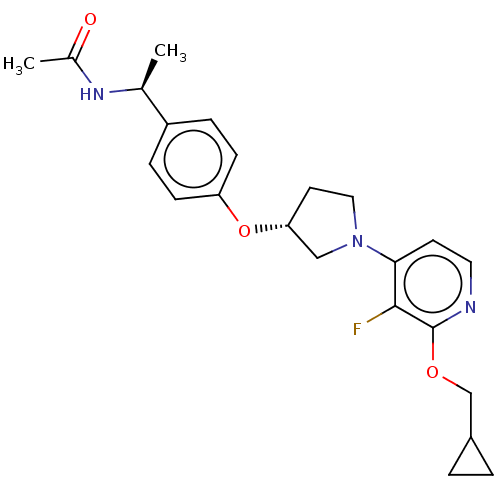 (US8877741, 9.51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138920 (US8877741, 9.56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138921 (US8877741, 9.57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 485 total ) | Next | Last >> |