

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158492 (US9029401, 2318) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158497 (US9029401, 2578) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158496 (US9029401, 2577) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50436435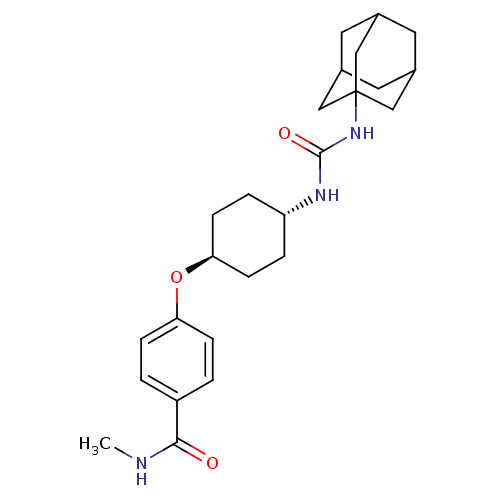 (CHEMBL2397139 | US9029401, 2225) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158488 (US9029401, 2278 (t-CUPM)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158486 (US9029401, 2228) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50436431 (CHEMBL2397149 | US9029401, 2576) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158493 (US9029401, 2319 (c-CUPM)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158491 (US9029401, 2315) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158498 (US9029401, 2579) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50436432 (CHEMBL2397136 | US9029401, 2287) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158485 (US10383835, Compound 2228 | US9029401, 2227) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158484 (US9029401, 2221) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158499 (US9029401, 2580) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50436441 (CHEMBL2397143 | US9029401, 2575) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50436438 (CHEMBL2397146 | US9029401, 2316) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158487 (US10383835, Compound 2226 | US9029401, 2253) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158500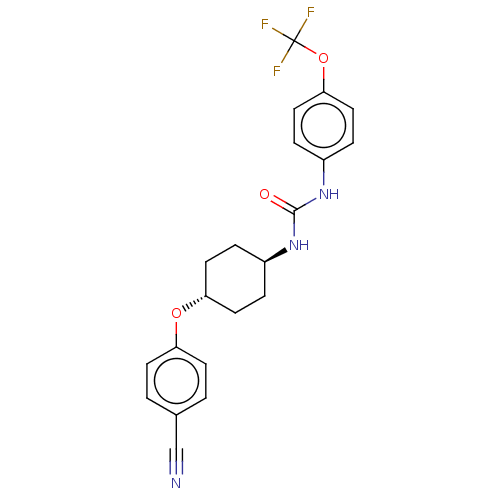 (US9029401, 2581) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158495 (US9029401, 2574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158480 (US9029401, 1686) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158481 (US9029401, 1728 (t-TUCB)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158489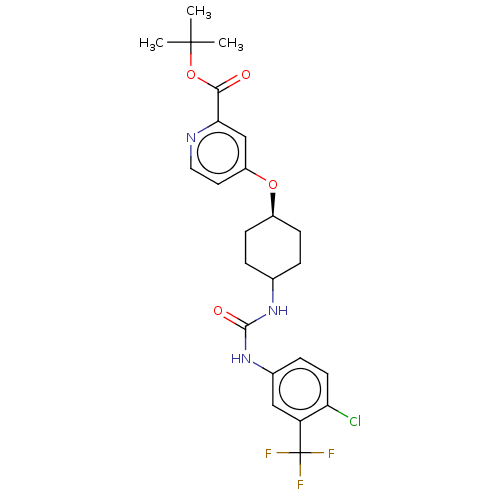 (US9029401, 2280) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158494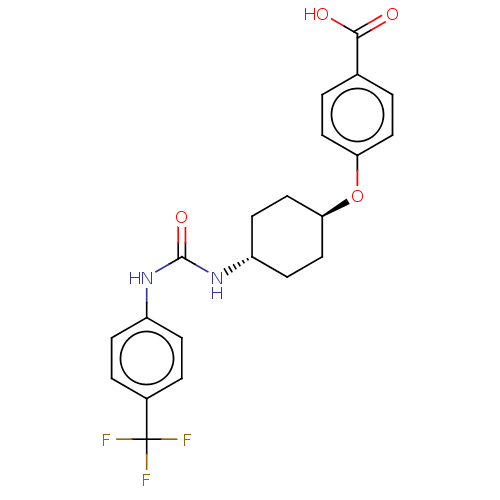 (US9029401, 2372) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217448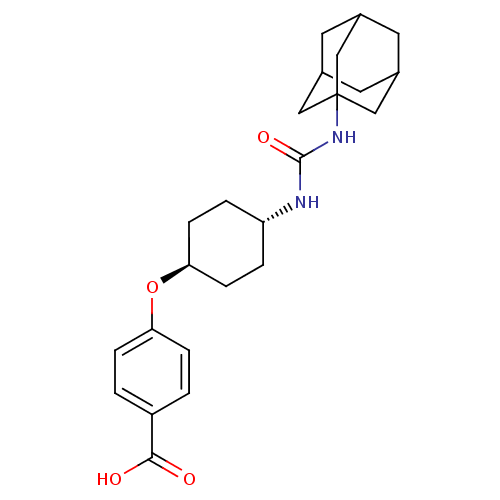 (CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158479 (US9029401, 1612) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158482 (US9029401, 2084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158490 (US9029401, 2288) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158483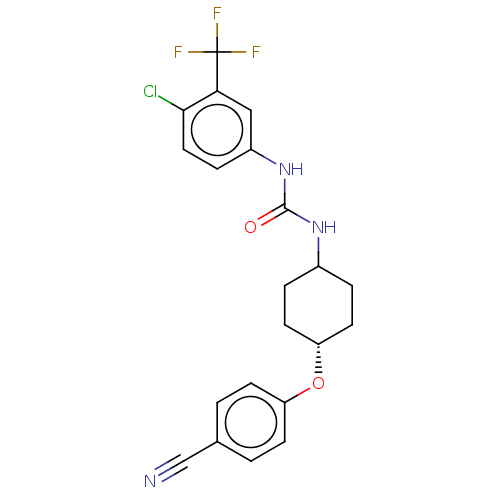 (US9029401, 2182) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50436431 (CHEMBL2397149 | US9029401, 2576) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158488 (US9029401, 2278 (t-CUPM)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50436438 (CHEMBL2397146 | US9029401, 2316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 175 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158493 (US9029401, 2319 (c-CUPM)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158485 (US10383835, Compound 2228 | US9029401, 2227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158489 (US9029401, 2280) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158488 (US9029401, 2278 (t-CUPM)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM50436438 (CHEMBL2397146 | US9029401, 2316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158493 (US9029401, 2319 (c-CUPM)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158484 (US9029401, 2221) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158485 (US10383835, Compound 2228 | US9029401, 2227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158495 (US9029401, 2574) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158484 (US9029401, 2221) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158478 (US10189853, sunitinib | US9029401, Sunitinib | US9...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50436435 (CHEMBL2397139 | US9029401, 2225) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM50436432 (CHEMBL2397136 | US9029401, 2287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158486 (US9029401, 2228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158481 (US9029401, 1728 (t-TUCB)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158482 (US9029401, 2084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158489 (US9029401, 2280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50436441 (CHEMBL2397143 | US9029401, 2575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158494 (US9029401, 2372) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158491 (US9029401, 2315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158478 (US10189853, sunitinib | US9029401, Sunitinib | US9...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Regents of the University of California US Patent | Assay Description See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay. | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM158490 (US9029401, 2288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM158490 (US9029401, 2288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50436432 (CHEMBL2397136 | US9029401, 2287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Regents of the University of California US Patent | Assay Description Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ... | US Patent US9029401 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||