

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 [D835H] (Homo sapiens (Human)) | BDBM247371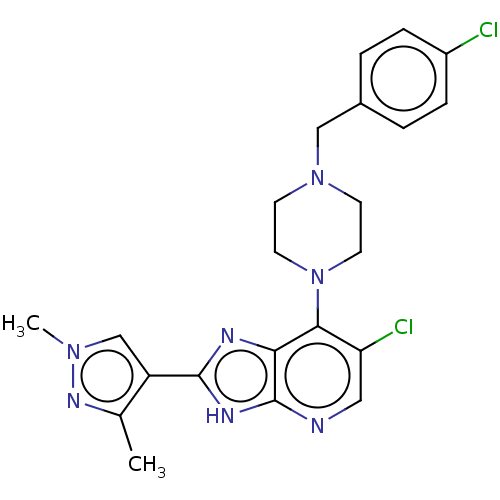 (US9447092, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [R834Q] (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247368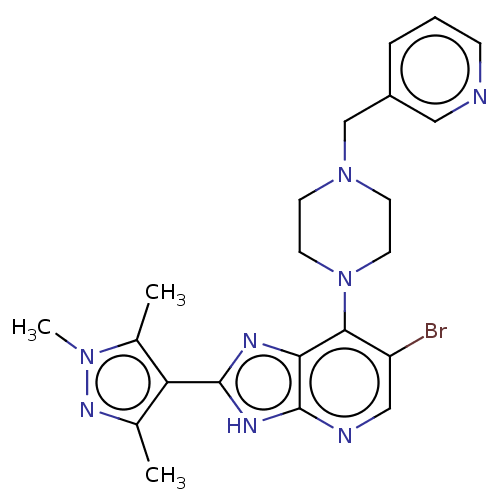 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247367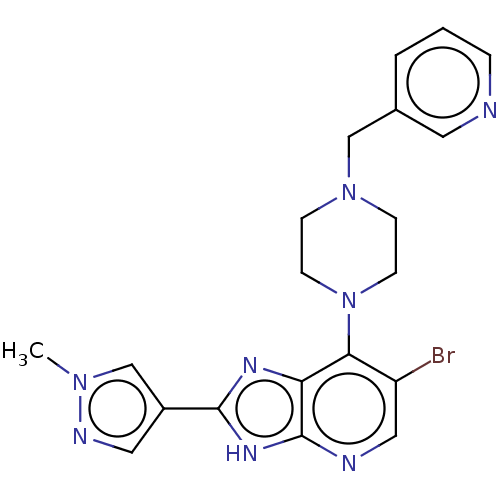 (US9447092, Comparator 1, Example 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247369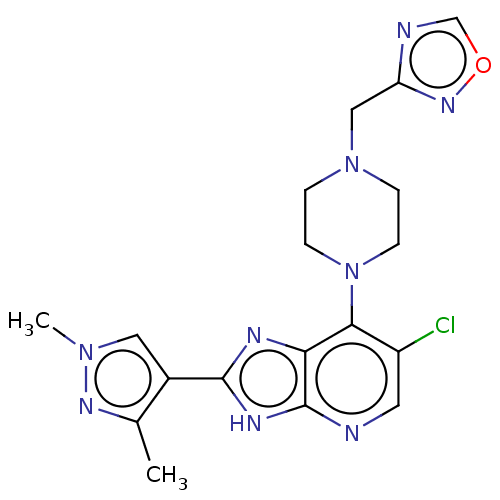 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247370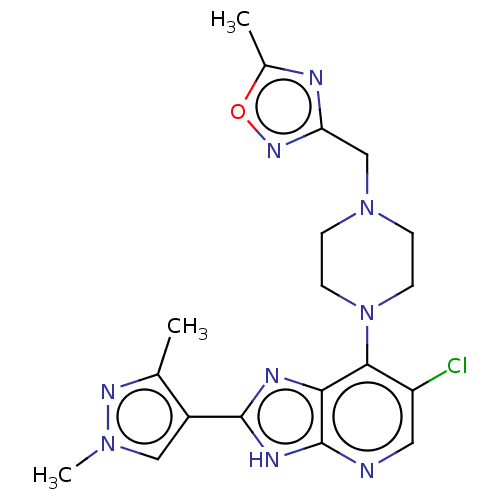 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [K663Q] (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [N841I] (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM247367 (US9447092, Comparator 1, Example 56) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247369 (US9447092, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM247371 (US9447092, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM247370 (US9447092, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM247368 (US9447092, Comparator 2, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED US Patent | Assay Description All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ... | US Patent US9447092 (2016) BindingDB Entry DOI: 10.7270/Q2PV6J9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||