

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088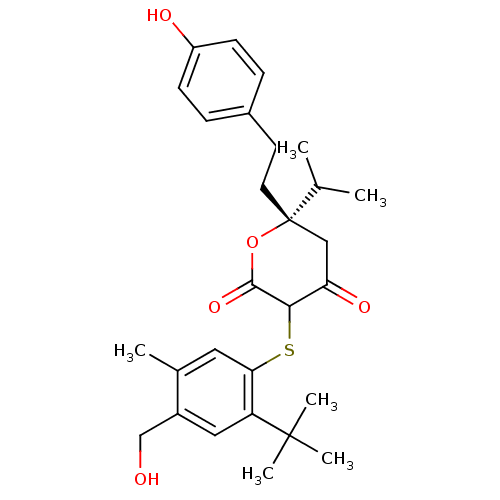 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50216785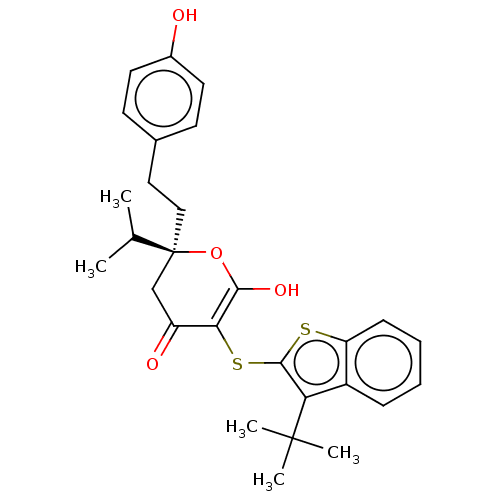 (CHEMBL61756) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50216785 (CHEMBL61756) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079704 (3-(3-tert-Butyl-benzo[b]thiophen-2-ylsulfanyl)-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2512 (3-[(2-tert-butyl-5-methylphenyl)sulfanyl]-4-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079697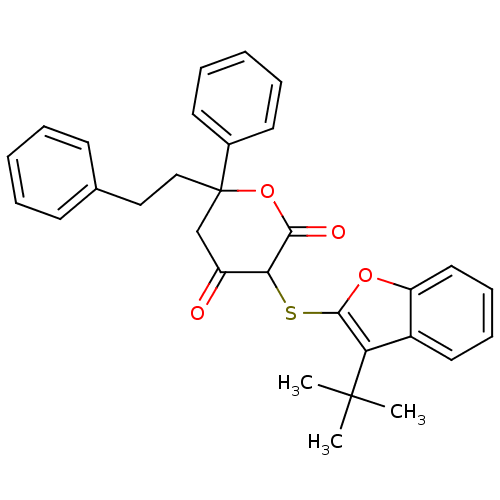 (3-(3-tert-Butyl-benzofuran-2-ylsulfanyl)-4-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079704 (3-(3-tert-Butyl-benzo[b]thiophen-2-ylsulfanyl)-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079702 (3-(3-tert-Butyl-5,6,7,8-tetrahydro-naphthalen-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079707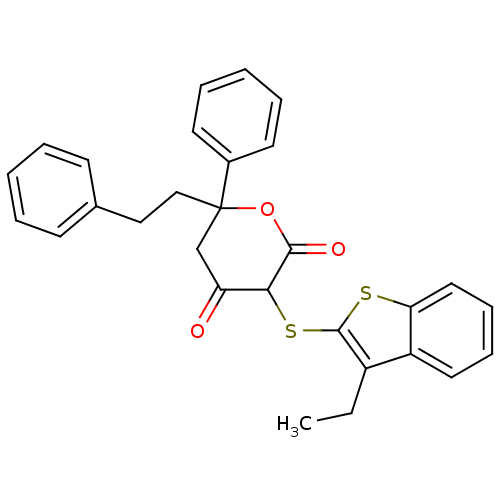 (3-(3-Ethyl-benzo[b]thiophen-2-ylsulfanyl)-4-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2512 (3-[(2-tert-butyl-5-methylphenyl)sulfanyl]-4-hydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079698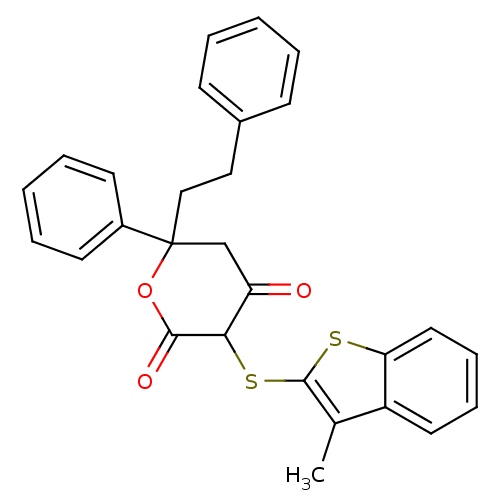 (4-Hydroxy-3-(3-methyl-benzo[b]thiophen-2-ylsulfany...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079701 (3-(6-tert-Butyl-indan-5-ylsulfanyl)-4-hydroxy-6-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079701 (3-(6-tert-Butyl-indan-5-ylsulfanyl)-4-hydroxy-6-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079709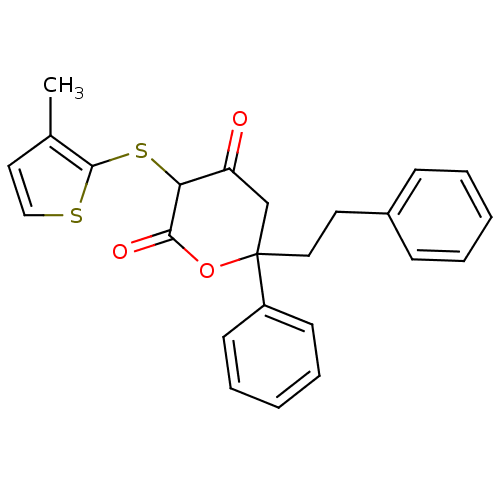 (4-Hydroxy-3-(3-methyl-thiophen-2-ylsulfanyl)-6-phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079697 (3-(3-tert-Butyl-benzofuran-2-ylsulfanyl)-4-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079705 (3-(2-tert-Butyl-benzofuran-3-ylsulfanyl)-4-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079705 (3-(2-tert-Butyl-benzofuran-3-ylsulfanyl)-4-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079703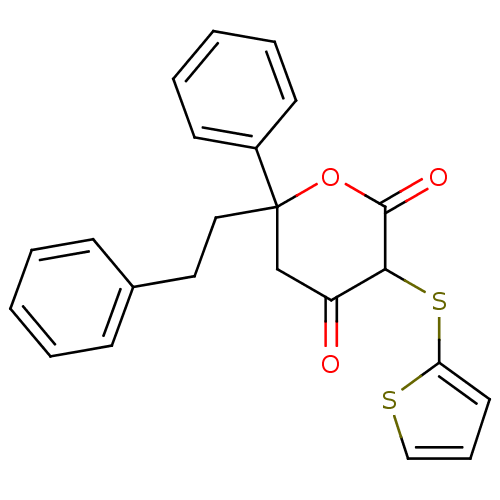 (4-Hydroxy-6-phenethyl-6-phenyl-3-(thiophen-2-ylsul...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079708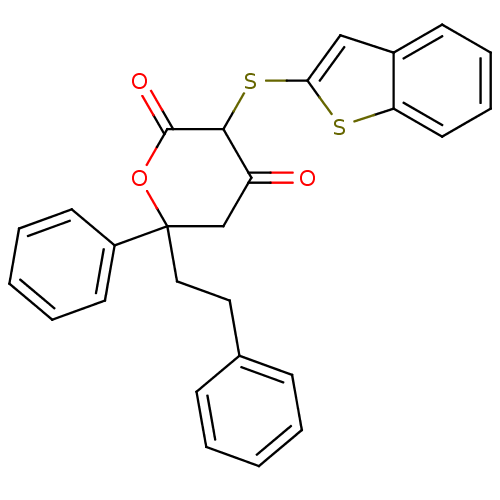 (3-(Benzo[b]thiophen-2-ylsulfanyl)-4-hydroxy-6-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 363 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50079699 (3-(1-tert-Butyl-1H-benzoimidazol-2-ylsulfanyl)-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 505 | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||