

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50029559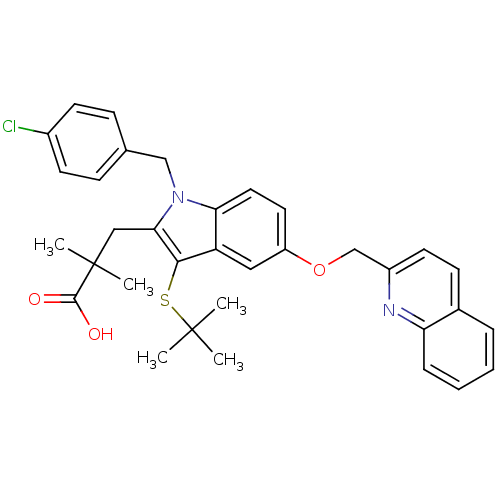 (2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080255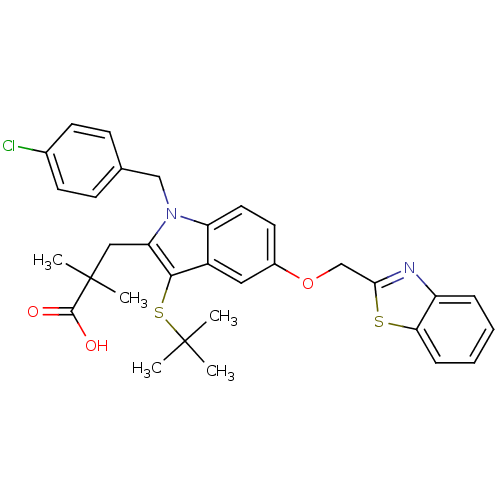 (3-[5-(Benzothiazol-2-ylmethoxy)-3-tert-butylsulfan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080243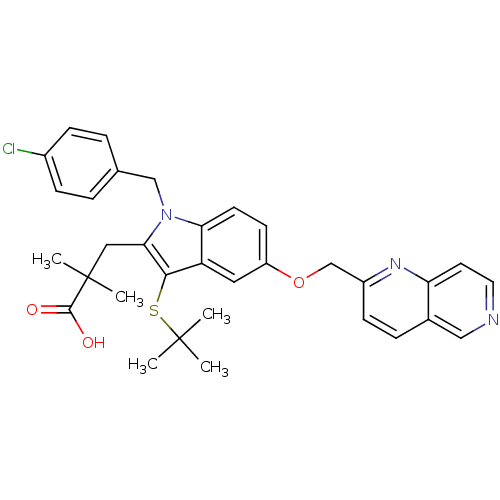 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-([1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080233 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(6-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080261 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-([1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080247 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080250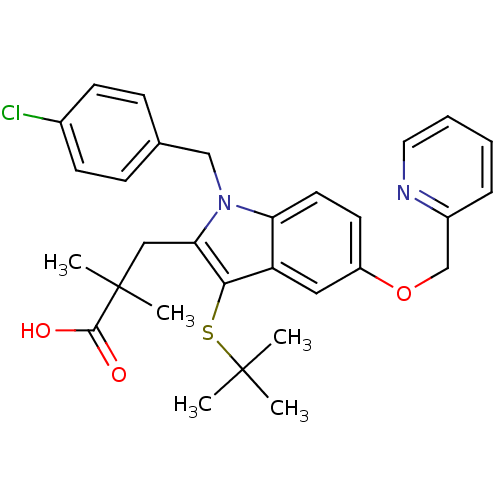 (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080258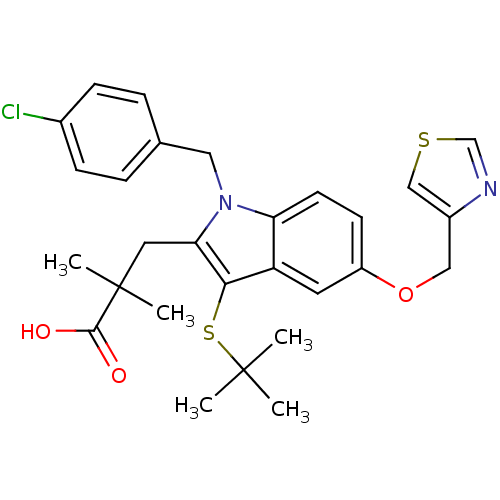 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080248 (3-[5-(Benzooxazol-2-ylmethoxy)-3-tert-butylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50403715 (CHEMBL2114162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-Lipoxygenase activating protein (FLAP) by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080251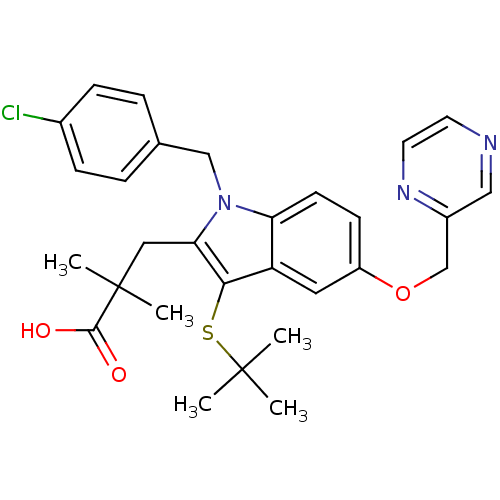 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080249 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(5,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080256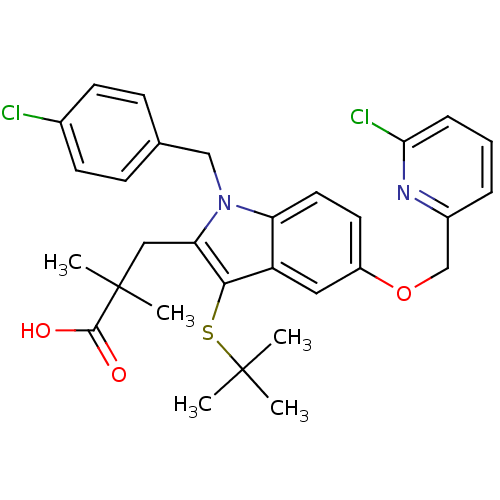 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(6-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080238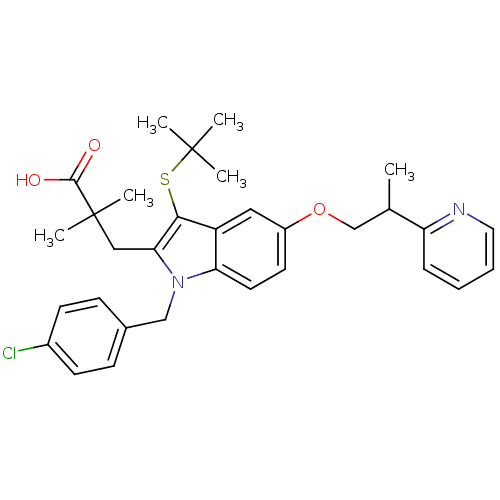 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080254 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080253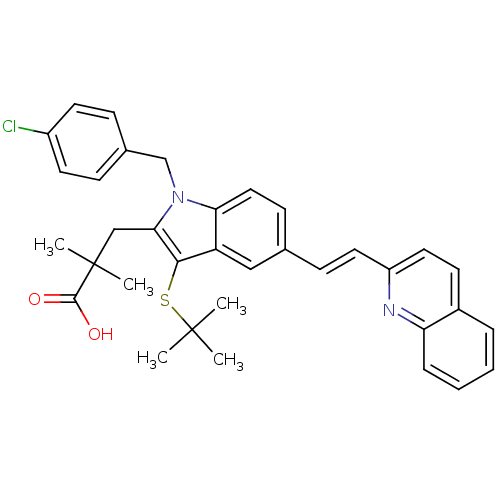 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-((E)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50217046 (CHEMBL3350287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080242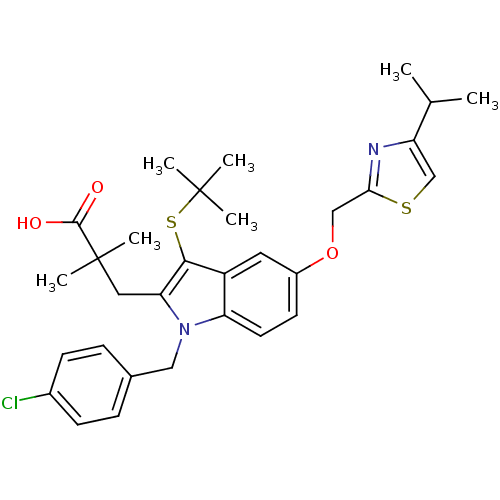 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(4-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080245 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080240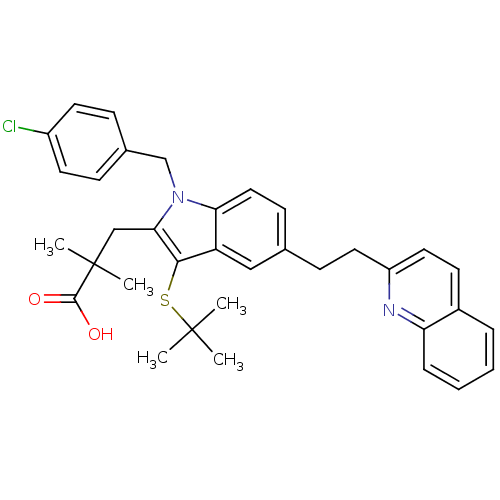 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(2-q...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080241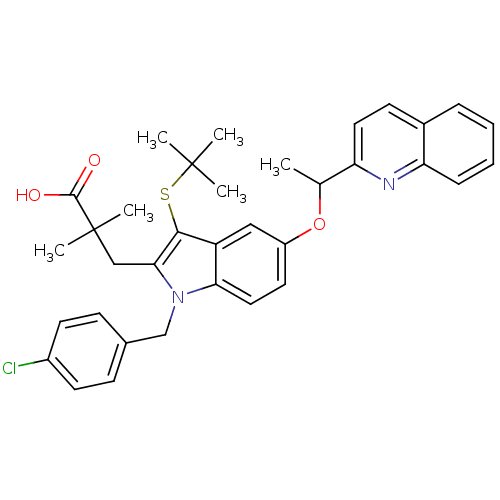 ((+)-3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080237 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080236 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080252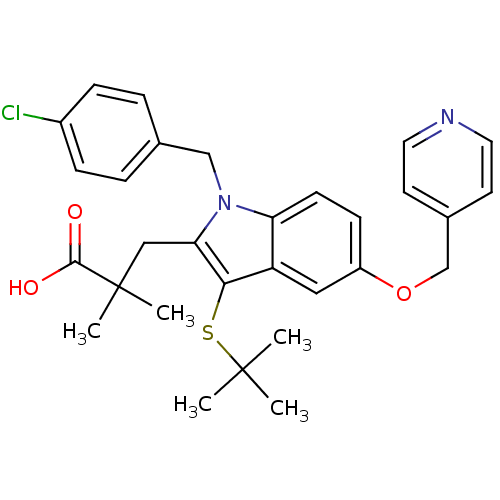 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080246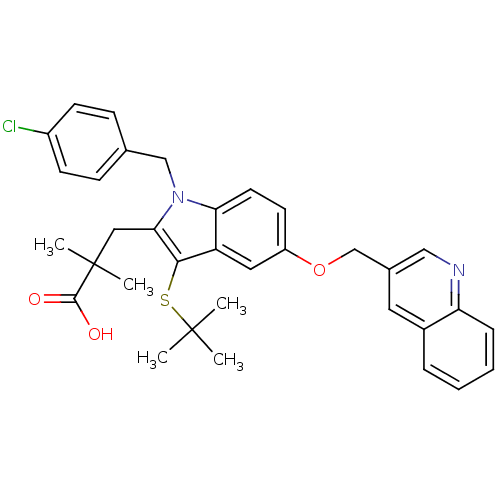 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080234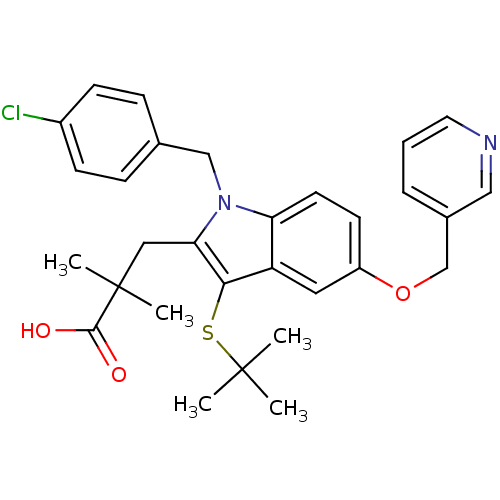 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080239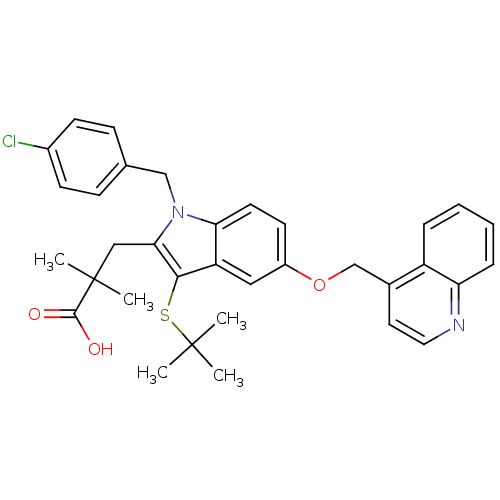 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080235 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080260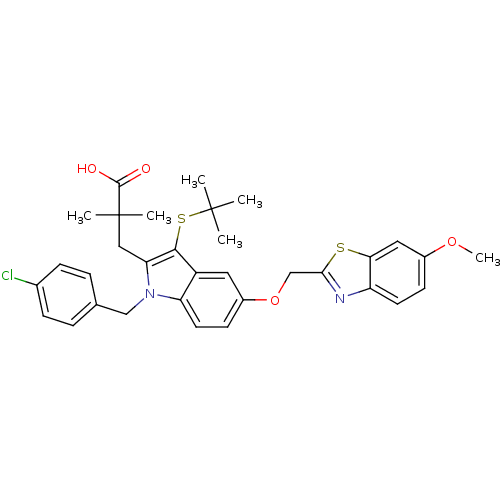 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(6-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080259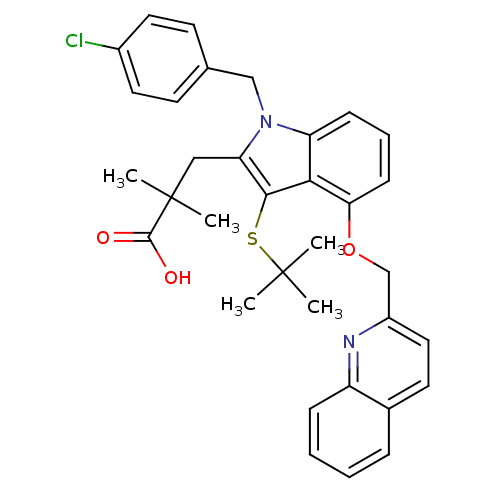 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-4-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080241 ((+)-3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080241 ((+)-3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080244 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(5-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080257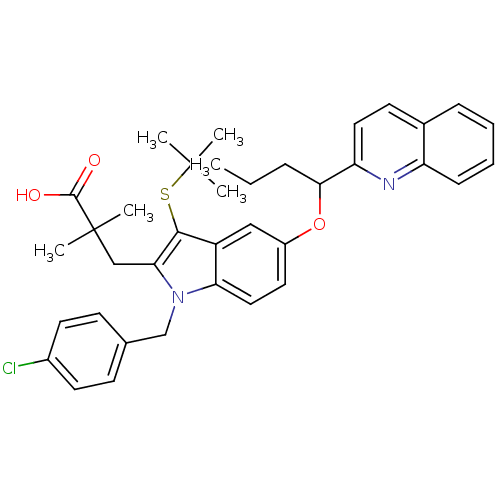 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(1-q...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080263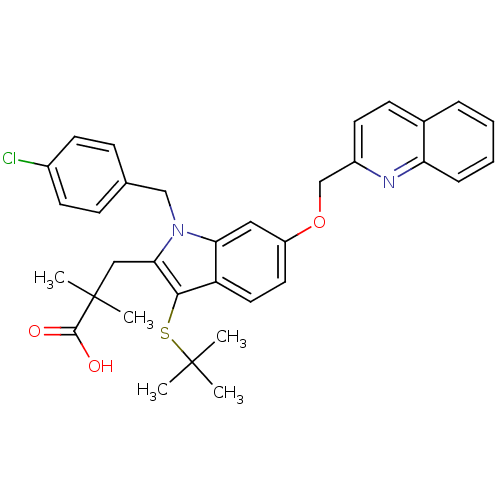 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-6-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50080262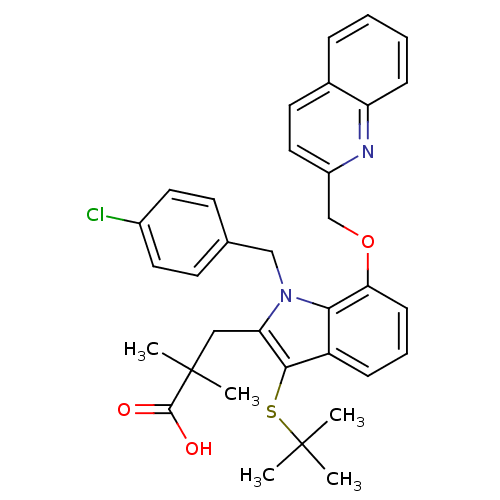 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-7-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Measuring the affinity of leukotriene synthesis inhibitor for 5-lipoxygenase activating protein by using [125I]-L-691,831 as radioligand. | Bioorg Med Chem Lett 9: 2391-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||