

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093056 ((S)-N-Benzo[1,3]dioxol-5-ylmethyl-4-cyclohexyl-3-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093057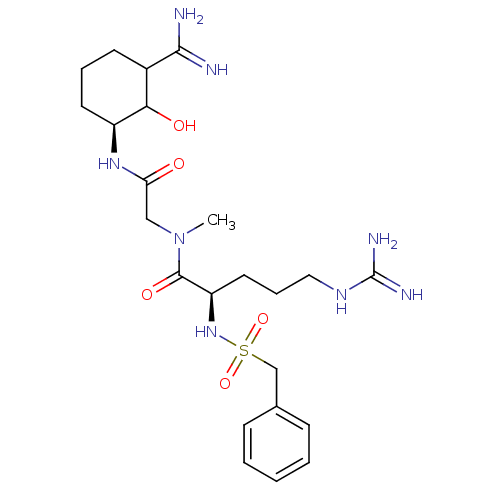 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093062 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093066 (((S)-4-Cyclohexyl-3-{2-[((R)-5-guanidino-2-phenylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093061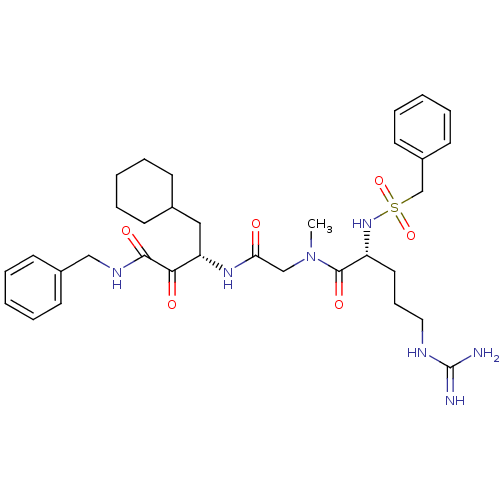 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093057 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against prothrombinase (PTase) complex | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093058 (((S)-3-{2-[((R)-5-Guanidino-2-phenylmethanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093060 (CHEMBL432159 | [(S)-3-{2-[((R)-5-Guanidino-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093065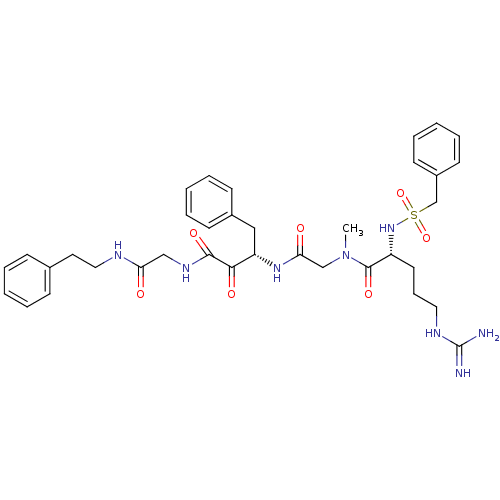 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against prothrombinase (PTase) complex | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093056 ((S)-N-Benzo[1,3]dioxol-5-ylmethyl-4-cyclohexyl-3-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093063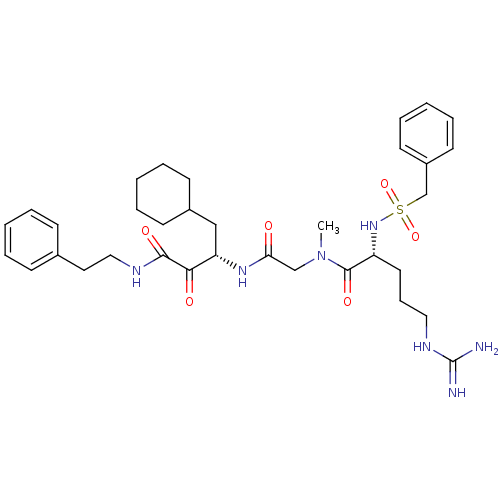 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093058 (((S)-3-{2-[((R)-5-Guanidino-2-phenylmethanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against prothrombinase (PTase) complex | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093065 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against prothrombinase (PTase) complex | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093060 (CHEMBL432159 | [(S)-3-{2-[((R)-5-Guanidino-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against prothrombinase (PTase) complex | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093064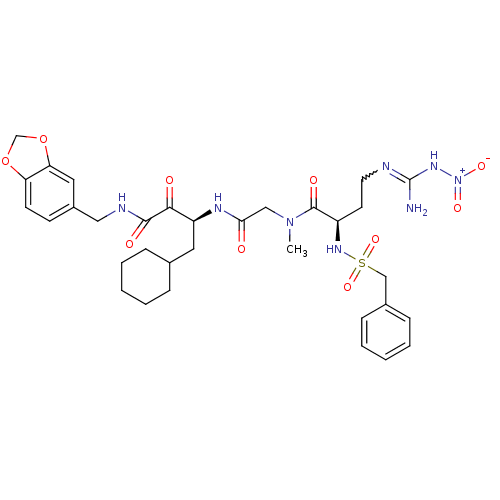 ((R)-5-Nitro-Guanidino-2-phenylmethanesulfonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093057 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093062 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50093059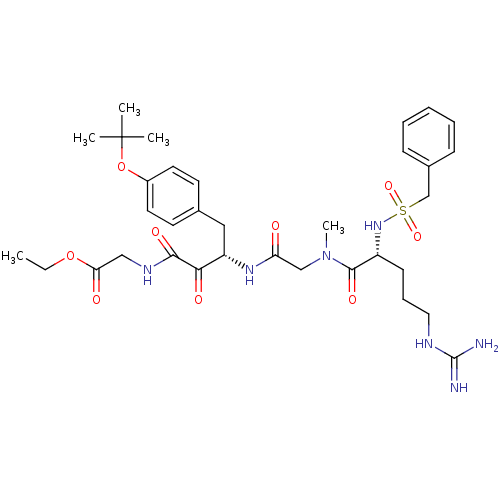 (((S)-4-(4-tert-Butoxy-phenyl)-3-{2-[((R)-5-guanidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093057 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093056 ((S)-N-Benzo[1,3]dioxol-5-ylmethyl-4-cyclohexyl-3-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093061 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093063 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093061 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093060 (CHEMBL432159 | [(S)-3-{2-[((R)-5-Guanidino-2-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093062 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093064 ((R)-5-Nitro-Guanidino-2-phenylmethanesulfonylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093056 ((S)-N-Benzo[1,3]dioxol-5-ylmethyl-4-cyclohexyl-3-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093057 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093056 ((S)-N-Benzo[1,3]dioxol-5-ylmethyl-4-cyclohexyl-3-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093058 (((S)-3-{2-[((R)-5-Guanidino-2-phenylmethanesulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093066 (((S)-4-Cyclohexyl-3-{2-[((R)-5-guanidino-2-phenylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093059 (((S)-4-(4-tert-Butoxy-phenyl)-3-{2-[((R)-5-guanidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093058 (((S)-3-{2-[((R)-5-Guanidino-2-phenylmethanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50093063 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Human trypsin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093065 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50093061 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against plasmin | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50093062 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against Thrombin (FIIa) | Bioorg Med Chem Lett 10: 2305-9 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||