

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094985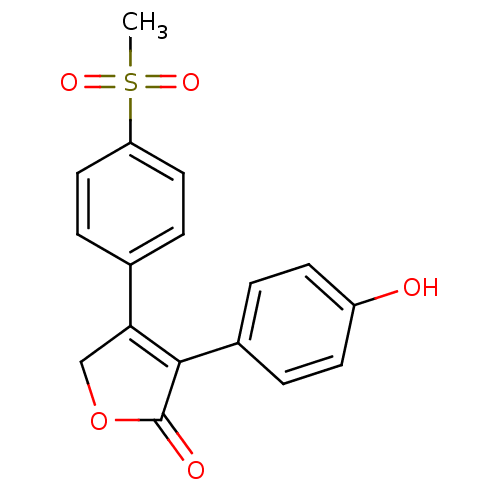 (3-(4-Hydroxy-phenyl)-4-(4-methanesulfonyl-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094985 (3-(4-Hydroxy-phenyl)-4-(4-methanesulfonyl-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094987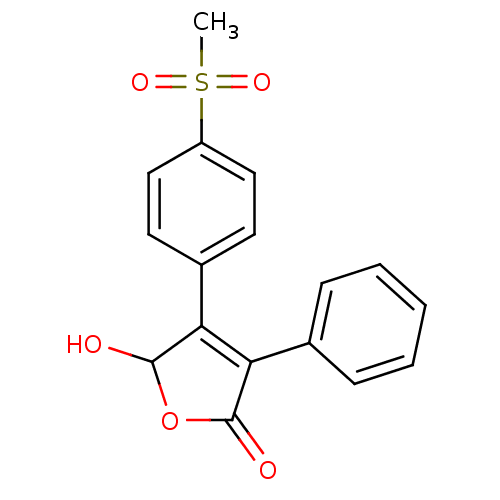 (5-Hydroxy Rofecoxib | 5-Hydroxy-4-(4-methanesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094983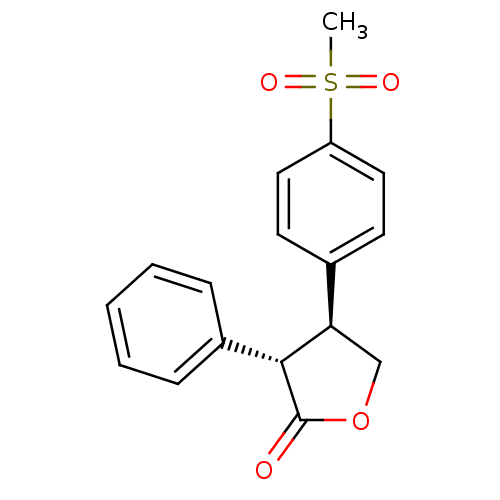 ((3S,4S)-4-(4-Methanesulfonyl-phenyl)-3-phenyl-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50451153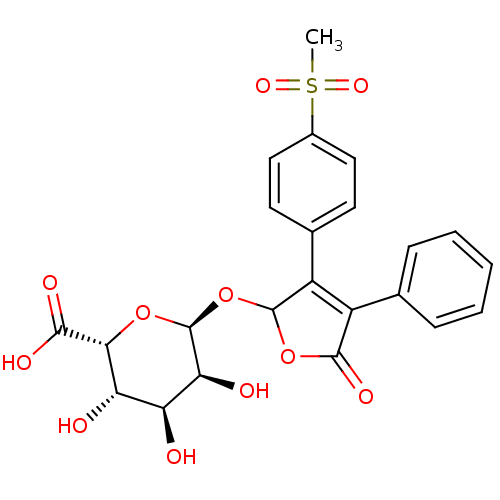 (CHEMBL2303696 | Glucuronide Conjugate Of 5-Hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50451154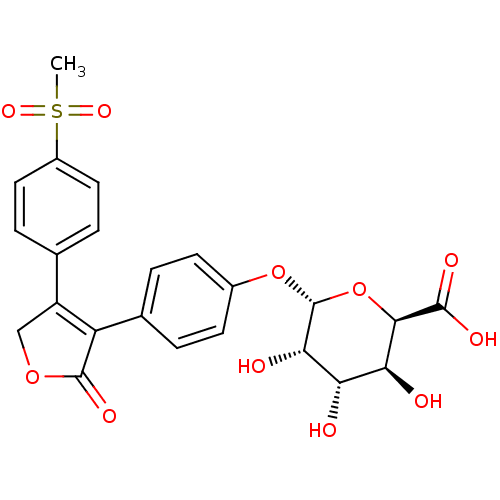 (CHEMBL2303697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094986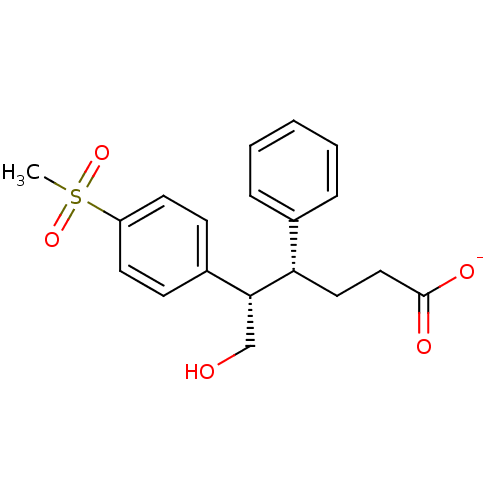 (CHEMBL327468 | Sodium; (4R,5S)-6-hydroxy-5-(4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094989 ((2S,3S)-4-Hydroxy-3-(4-methanesulfonyl-phenyl)-2-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094987 (5-Hydroxy Rofecoxib | 5-Hydroxy-4-(4-methanesulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094989 ((2S,3S)-4-Hydroxy-3-(4-methanesulfonyl-phenyl)-2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094988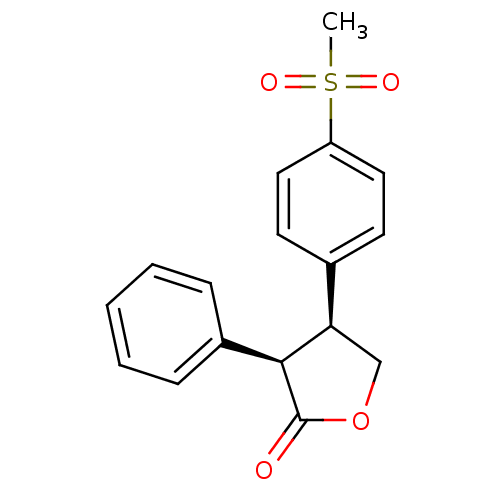 ((3R,4S)-4-(4-Methanesulfonyl-phenyl)-3-phenyl-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094986 (CHEMBL327468 | Sodium; (4R,5S)-6-hydroxy-5-(4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094988 ((3R,4S)-4-(4-Methanesulfonyl-phenyl)-3-phenyl-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094983 ((3S,4S)-4-(4-Methanesulfonyl-phenyl)-3-phenyl-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50451153 (CHEMBL2303696 | Glucuronide Conjugate Of 5-Hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50451154 (CHEMBL2303697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 1. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||