

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (MOUSE) | BDBM50370067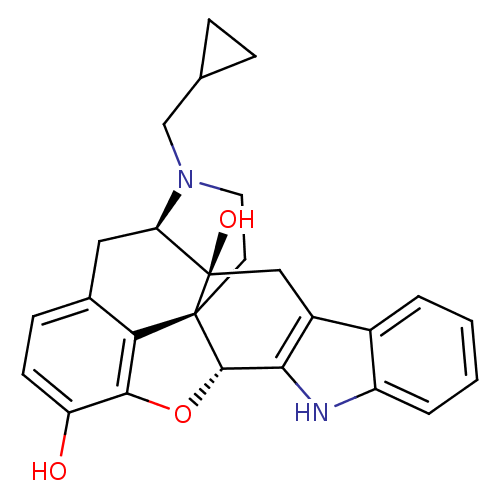 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50221416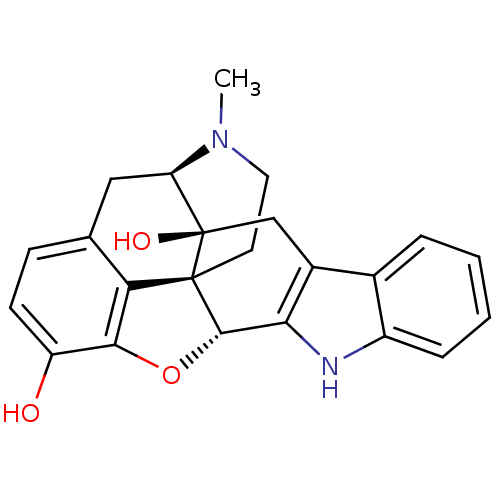 (22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098673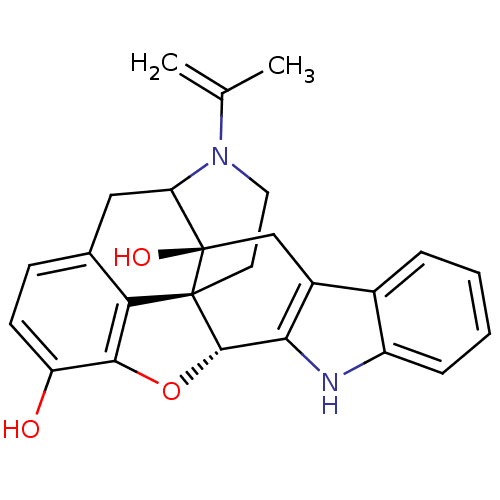 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098677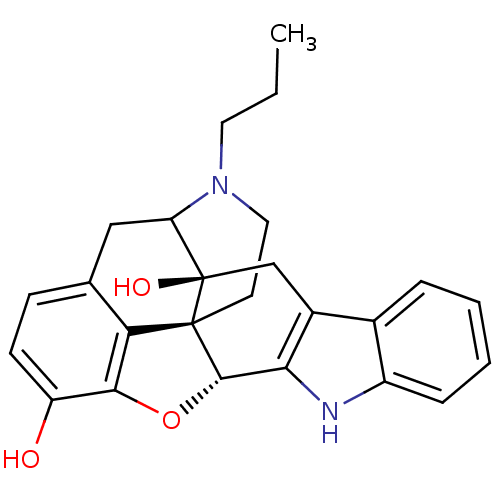 (17-propyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on SNC 80 stimulated [35S]GTP-gamma-S binding to delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098683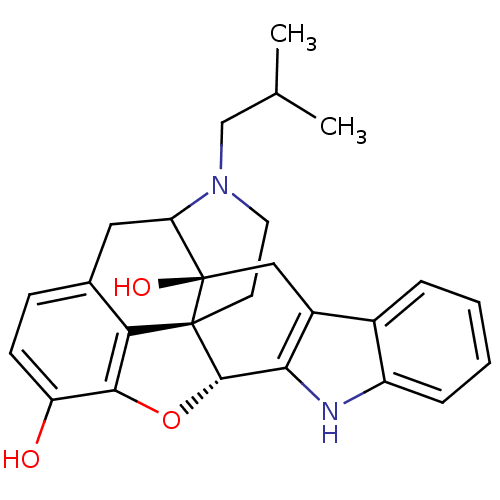 (17-(2-methylpropyl)-6,7-dehydro-4,5alpha-epoxy-3,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50370067 (CHEMBL1237164) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on U50,488H stimulated [35S]GTP-gamma-S binding to opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098673 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098676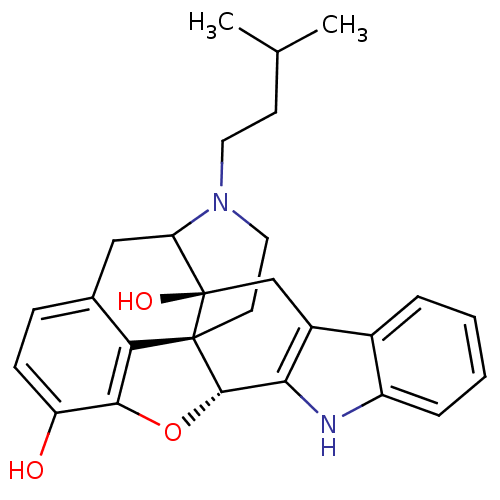 (17-(3-methylbutyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098681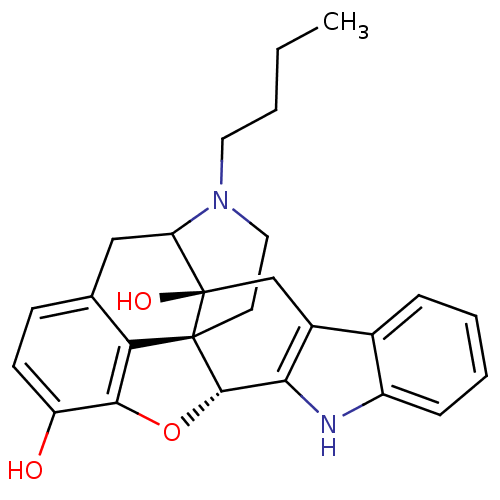 (17-butyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098675 (17-pentyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098679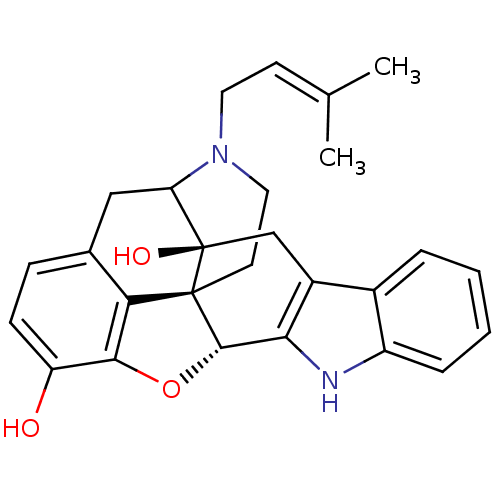 (17-(3-methyl-2-butenyl)-6,7-dehydro-4,5alpha-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098672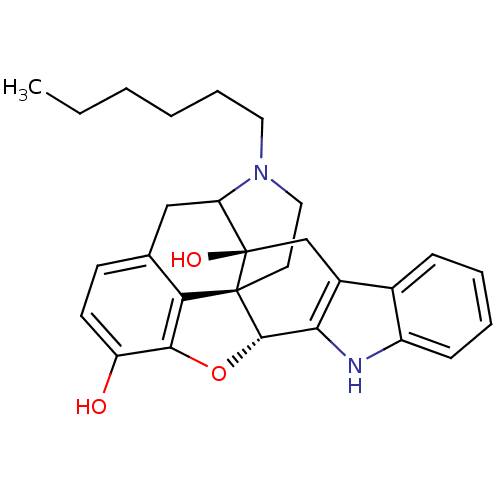 (17-hexyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370067 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50370067 (CHEMBL1237164) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098678 (17-(1-methylethyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221416 (22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50098682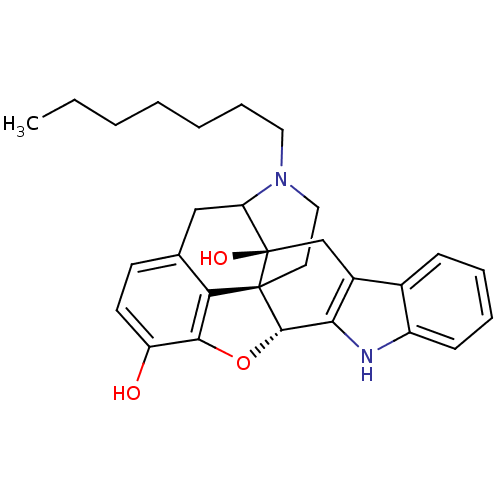 (17-heptyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DADLE at delta-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on U50,488H stimulated [35S]GTP-gamma-S binding to opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098673 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on agonist stimulated [35S]GTP-gamma-S binding on mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on DAMGO stimulated [35S]GTP-gamma-S binding to mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098677 (17-propyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098683 (17-(2-methylpropyl)-6,7-dehydro-4,5alpha-epoxy-3,1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098676 (17-(3-methylbutyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098677 (17-propyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098673 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on U50,488H stimulated [35S]GTP-gamma-S binding to opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098681 (17-butyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity of compound on U50,488H stimulated [35S]GTP-gamma-S binding to opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098674 ((E)-17-(2-butenyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098681 (17-butyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098676 (17-(3-methylbutyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098675 (17-pentyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098683 (17-(2-methylpropyl)-6,7-dehydro-4,5alpha-epoxy-3,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098679 (17-(3-methyl-2-butenyl)-6,7-dehydro-4,5alpha-epoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098675 (17-pentyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098680 (17-ethyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098672 (17-hexyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098679 (17-(3-methyl-2-butenyl)-6,7-dehydro-4,5alpha-epoxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098673 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098682 (17-heptyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098672 (17-hexyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098678 (17-(1-methylethyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098678 (17-(1-methylethyl)-6,7-dehydro-4,5alpha-epoxy-3,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50098682 (17-heptyl-6,7-dehydro-4,5alpha-epoxy-3,14-dihydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 at opioid receptor kappa 1 | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098673 (17-(2-methyl-2-propenyl)-6,7-dehydro-4,5alpha-epox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO at mu-opioid receptor | J Med Chem 44: 1471-4 (2001) BindingDB Entry DOI: 10.7270/Q2PK0GVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||