 Found 17 hits of Enzyme Inhibition Constant Data
Found 17 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 8 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity towards L100I mutant HIV-1 reverse transcriptase (as per ref 10 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 6 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2337
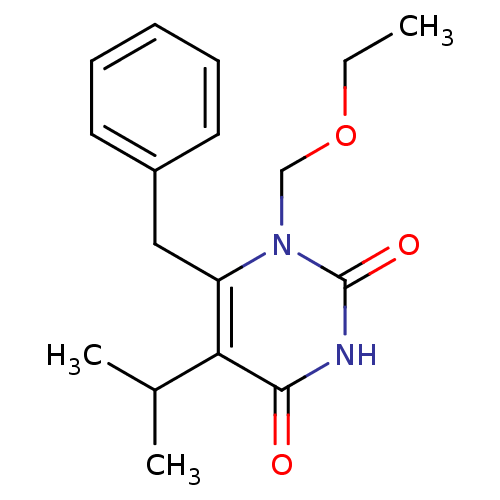
(6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...)Show InChI InChI=1S/C17H22N2O3/c1-4-22-11-19-14(10-13-8-6-5-7-9-13)15(12(2)3)16(20)18-17(19)21/h5-9,12H,4,10-11H2,1-3H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 12 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 6 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50279762
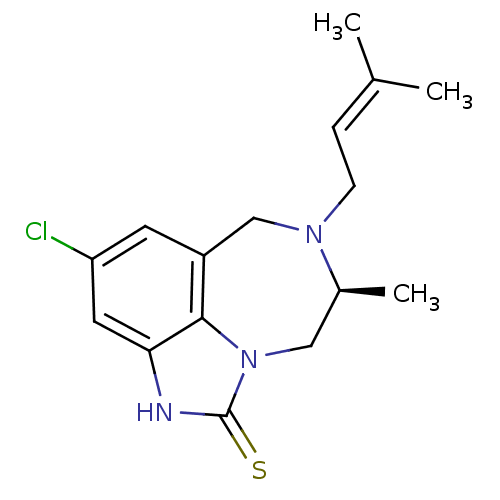
((5S)-9-chloro-5-methyl-6-(3-methylbut-2-enyl)-4,5,...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)cc(Cl)cc3[nH]c2=S |wD:1.0,(11.88,-16.19,;11.86,-14.64,;13.24,-13.98,;13.57,-12.47,;12.6,-11.27,;11.07,-11.28,;10.11,-12.49,;10.46,-13.99,;9.27,-14.97,;7.83,-14.43,;6.62,-15.39,;5.19,-14.85,;6.87,-16.91,;10.3,-9.95,;11.06,-8.61,;10.27,-7.28,;12.6,-8.61,;13.37,-9.95,;14.87,-10.27,;15.02,-11.79,;16.37,-12.56,)| Show InChI InChI=1S/C16H20ClN3S/c1-10(2)4-5-19-9-12-6-13(17)7-14-15(12)20(8-11(19)3)16(21)18-14/h4,6-7,11H,5,8-9H2,1-3H3,(H,18,21)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 8 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50105629

(CHEMBL54893 | N-(4-chloro-3-(3-methylbut-2-enyloxy...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl Show InChI InChI=1S/C17H18ClNO2S/c1-11(2)6-8-21-16-10-13(4-5-15(16)18)19-17(22)14-7-9-20-12(14)3/h4-7,9-10H,8H2,1-3H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50105629

(CHEMBL54893 | N-(4-chloro-3-(3-methylbut-2-enyloxy...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl Show InChI InChI=1S/C17H18ClNO2S/c1-11(2)6-8-21-16-10-13(4-5-15(16)18)19-17(22)14-7-9-20-12(14)3/h4-7,9-10H,8H2,1-3H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 7 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50279762

((5S)-9-chloro-5-methyl-6-(3-methylbut-2-enyl)-4,5,...)Show SMILES C[C@H]1Cn2c3c(CN1CC=C(C)C)cc(Cl)cc3[nH]c2=S |wD:1.0,(11.88,-16.19,;11.86,-14.64,;13.24,-13.98,;13.57,-12.47,;12.6,-11.27,;11.07,-11.28,;10.11,-12.49,;10.46,-13.99,;9.27,-14.97,;7.83,-14.43,;6.62,-15.39,;5.19,-14.85,;6.87,-16.91,;10.3,-9.95,;11.06,-8.61,;10.27,-7.28,;12.6,-8.61,;13.37,-9.95,;14.87,-10.27,;15.02,-11.79,;16.37,-12.56,)| Show InChI InChI=1S/C16H20ClN3S/c1-10(2)4-5-19-9-12-6-13(17)7-14-15(12)20(8-11(19)3)16(21)18-14/h4,6-7,11H,5,8-9H2,1-3H3,(H,18,21)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 7 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 12 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2337

(6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...)Show InChI InChI=1S/C17H22N2O3/c1-4-22-11-19-14(10-13-8-6-5-7-9-13)15(12(2)3)16(20)18-17(19)21/h5-9,12H,4,10-11H2,1-3H3,(H,18,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity towards wild type HIV-1 reverse transcriptase (as per ref 10 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 12 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 7 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 6 in the article) |
Bioorg Med Chem Lett 11: 2799-802 (2001)
BindingDB Entry DOI: 10.7270/Q2J103P4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data