

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM23400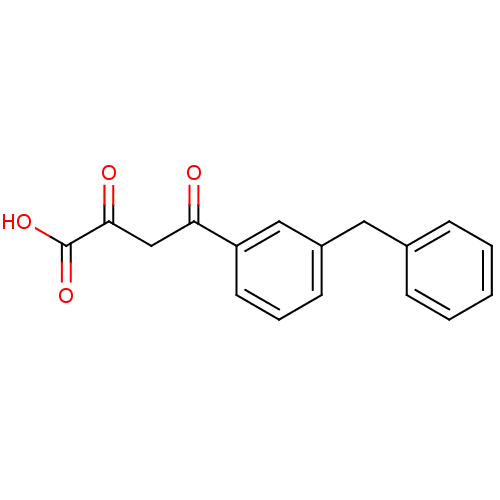 ((2Z)-4-(3-benzylphenyl)-2-hydroxy-4-oxobut-2-enoic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123468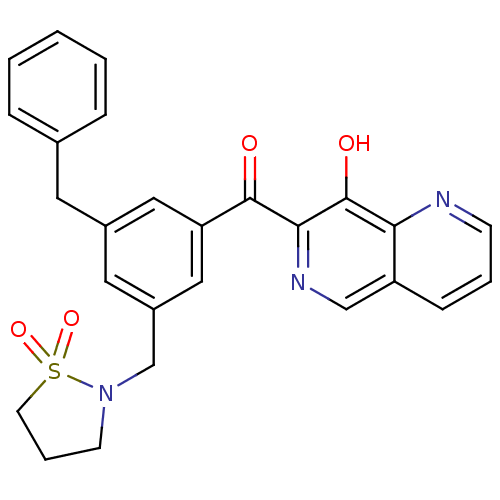 (CHEMBL32865 | [3-Benzyl-5-(1,1-dioxo-1lambda 6 -is...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123469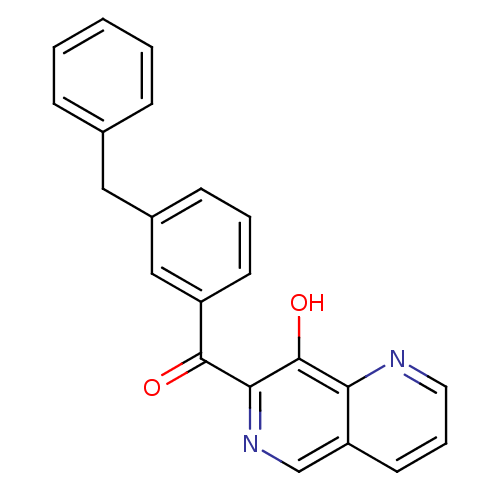 ((3-Benzyl-phenyl)-(8-hydroxy-[1,6]naphthyridin-7-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123471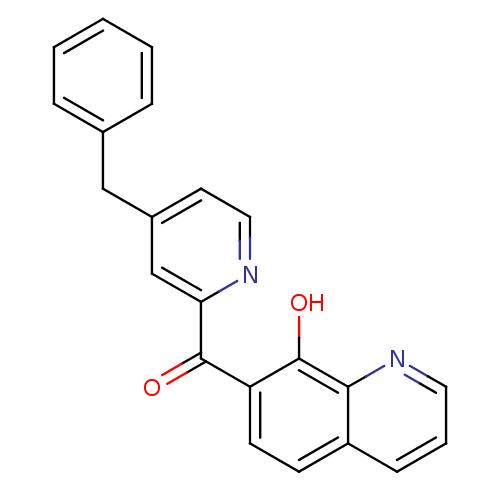 ((4-Benzyl-pyridin-2-yl)-(8-hydroxy-quinolin-7-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123470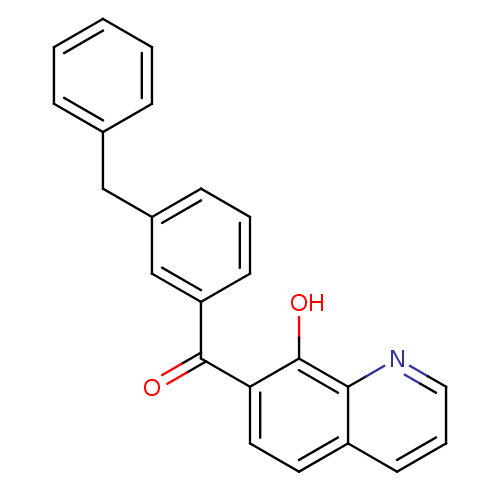 ((3-Benzyl-phenyl)-(8-hydroxy-quinolin-7-yl)-methan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123472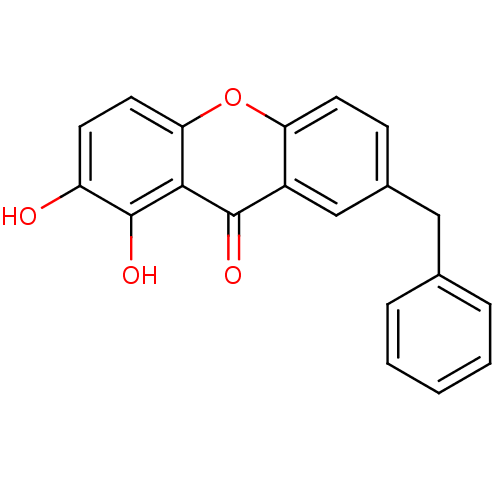 (7-Benzyl-1,2-dihydroxy-xanthen-9-one | CHEMBL28809...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123467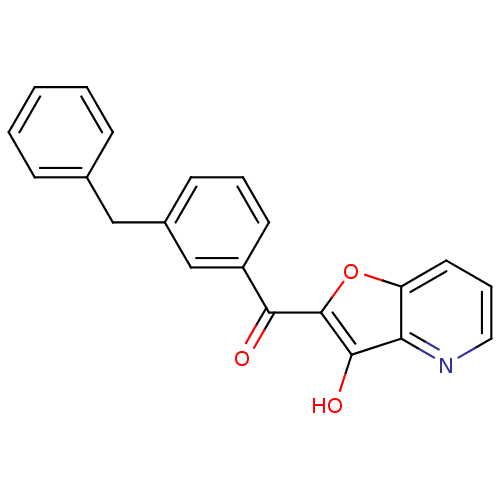 ((3-Benzyl-phenyl)-(3-hydroxy-furo[3,2-b]pyridin-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the strand transfer of the integration process catalyzed by HIV integrase | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123468 (CHEMBL32865 | [3-Benzyl-5-(1,1-dioxo-1lambda 6 -is...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >800 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Potency of compound was tested against HIV-1- containing integrase mutations (T66I, S153Y) | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50123468 (CHEMBL32865 | [3-Benzyl-5-(1,1-dioxo-1lambda 6 -is...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Potency of compound was tested against HIV-1- containing integrase mutations (NL4-3) | J Med Chem 46: 453-6 (2003) Article DOI: 10.1021/jm025553u BindingDB Entry DOI: 10.7270/Q24T6HRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||