

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126275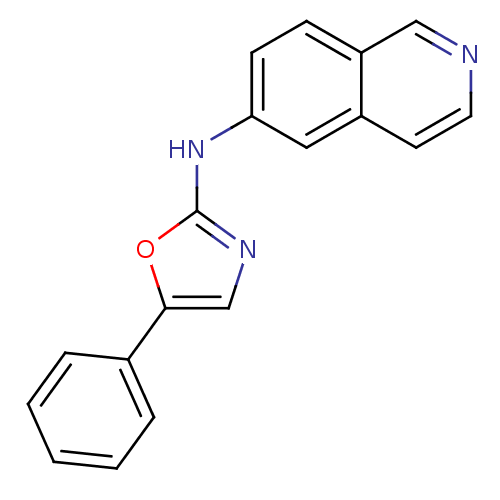 (CHEMBL280793 | Isoquinolin-6-yl-(5-phenyl-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126276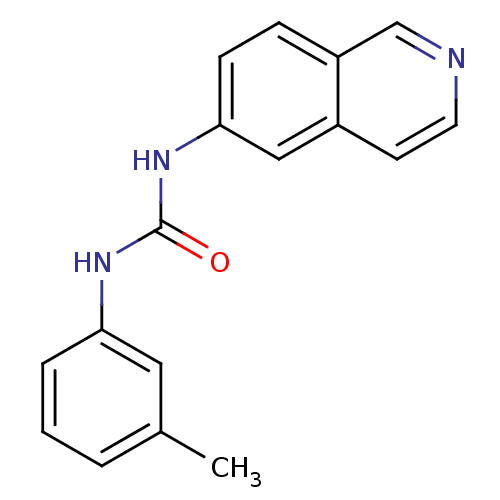 (1-Isoquinolin-6-yl-3-m-tolyl-urea | CHEMBL27948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126273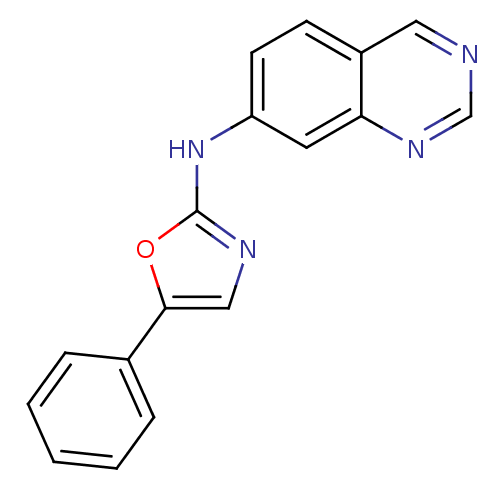 ((5-Phenyl-oxazol-2-yl)-quinazolin-7-yl-amine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126265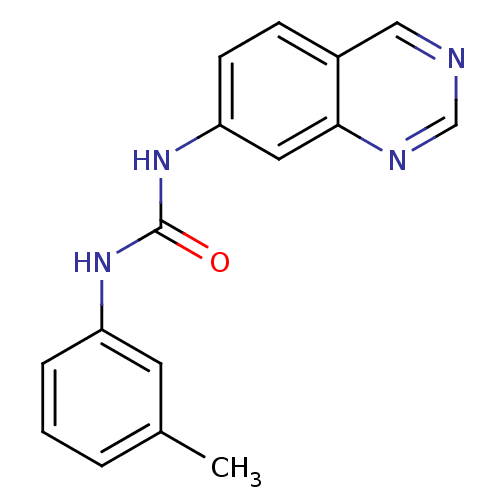 (1-Quinazolin-7-yl-3-m-tolyl-urea | CHEMBL26741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126283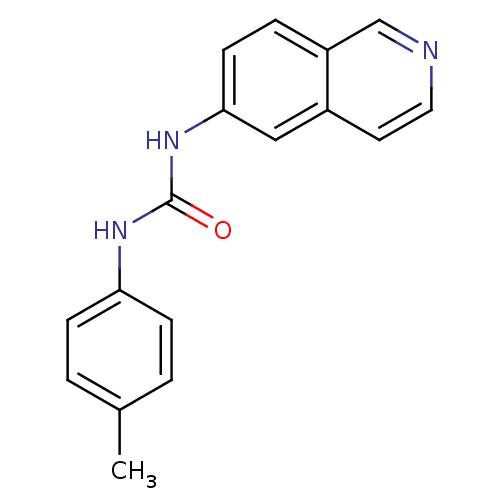 (1-Isoquinolin-6-yl-3-p-tolyl-urea | CHEMBL29927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126285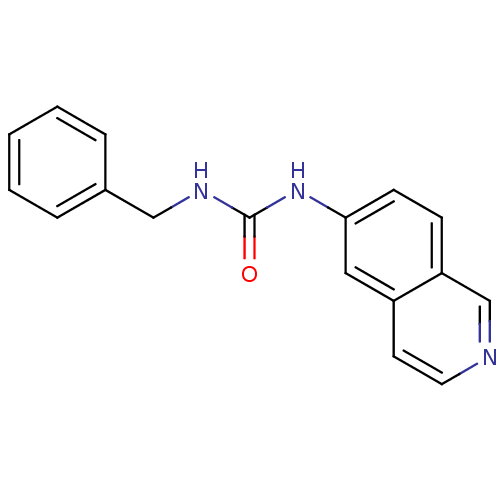 (1-Benzyl-3-isoquinolin-6-yl-urea | CHEMBL29926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126284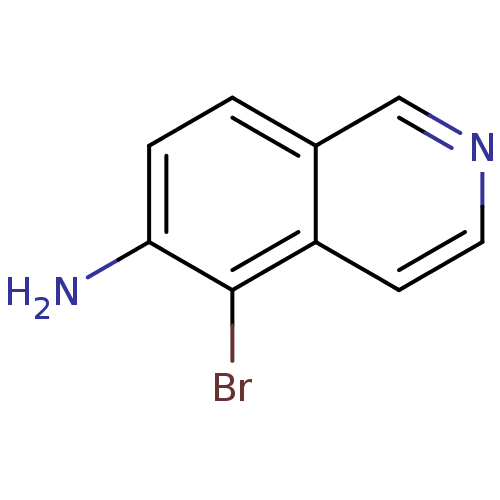 (5-Bromo-isoquinolin-6-ylamine | CHEMBL27011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126282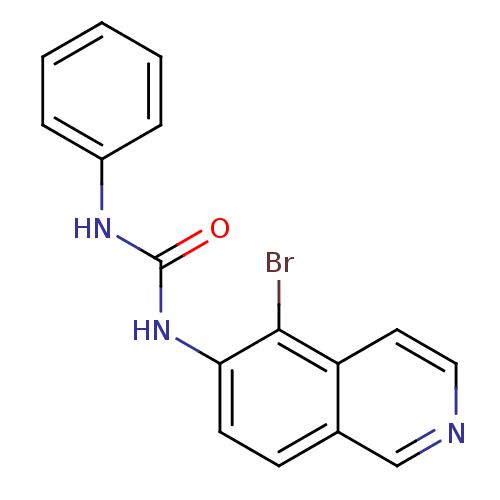 (1-(5-Bromo-isoquinolin-6-yl)-3-phenyl-urea | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126268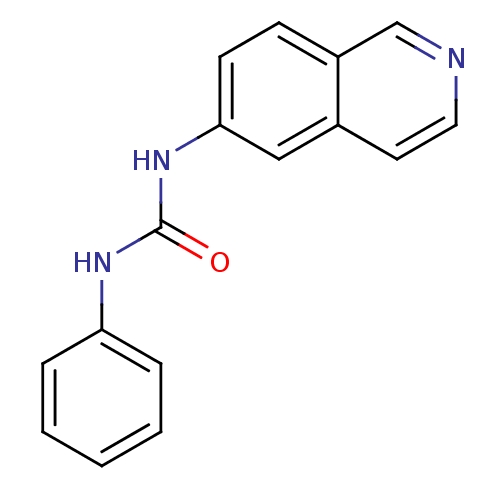 (1-Isoquinolin-6-yl-3-phenyl-urea | CHEMBL27529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126270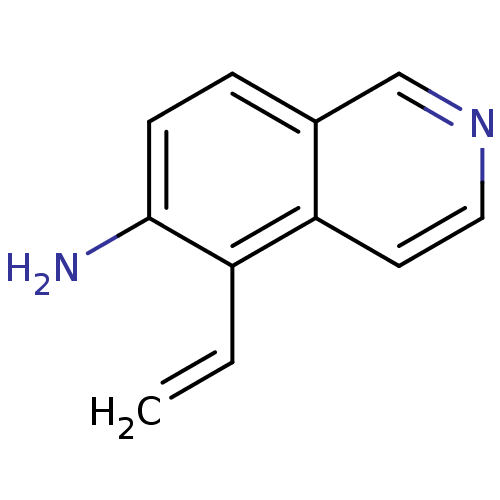 (5-Vinyl-isoquinolin-6-ylamine | CHEMBL26155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126287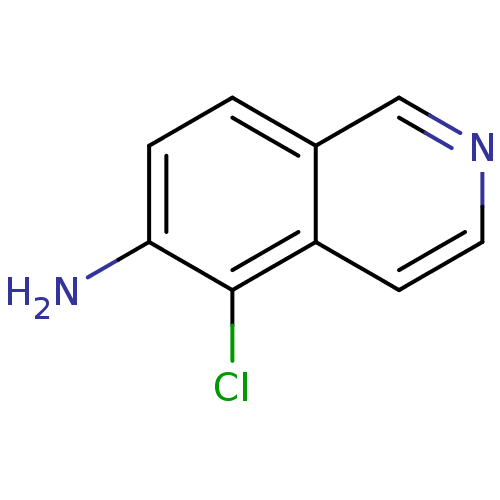 (5-Chloro-isoquinolin-6-ylamine | CHEMBL416020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126288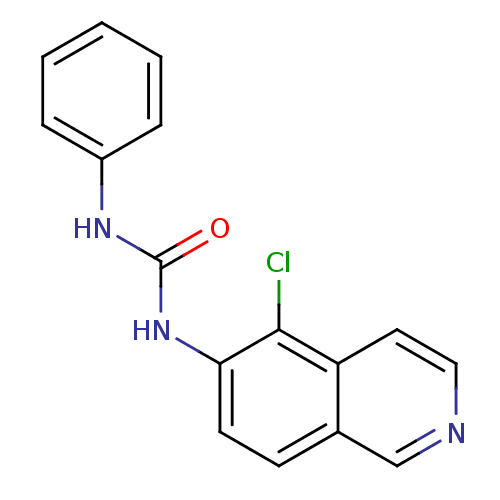 (1-(5-Chloro-isoquinolin-6-yl)-3-phenyl-urea | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126280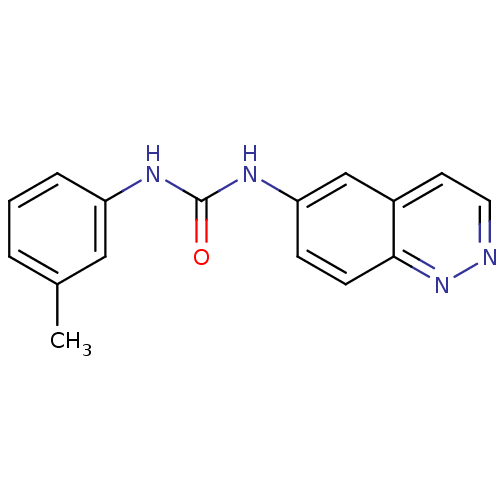 (1-Cinnolin-6-yl-3-m-tolyl-urea | CHEMBL26025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126278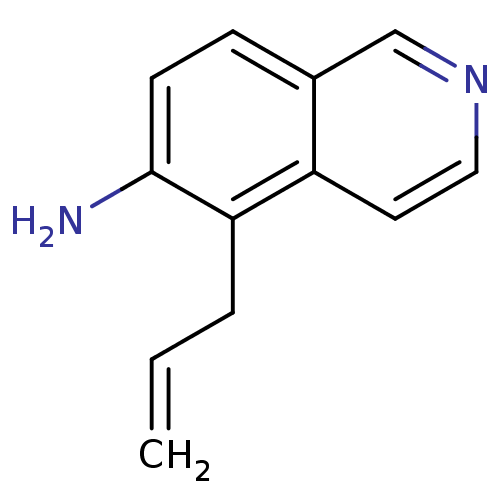 (5-Allyl-isoquinolin-6-ylamine | CHEMBL282102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126277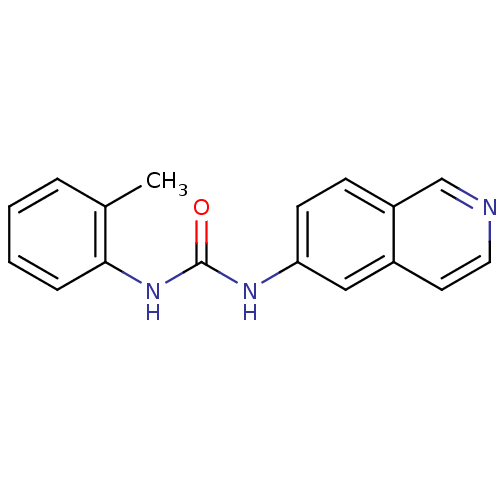 (1-Isoquinolin-6-yl-3-o-tolyl-urea | CHEMBL27528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126272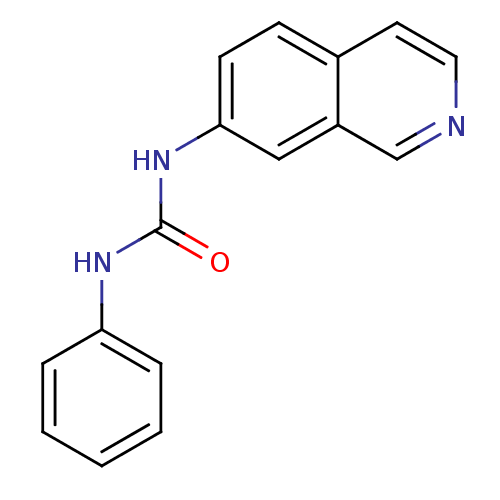 (1-Isoquinolin-7-yl-3-phenyl-urea | CHEMBL283016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126286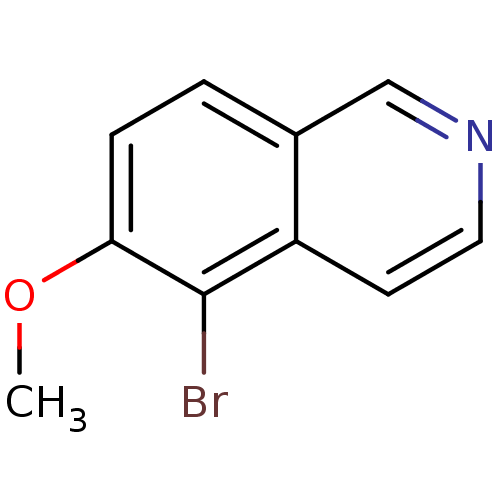 (5-Bromo-6-methoxy-isoquinoline | CHEMBL26259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126281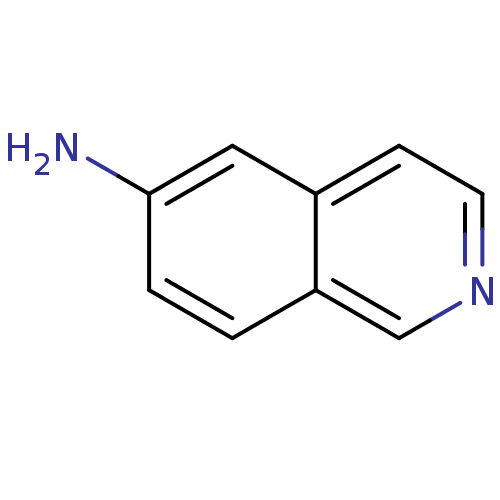 (CHEMBL28687 | Isoquinolin-6-ylamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126279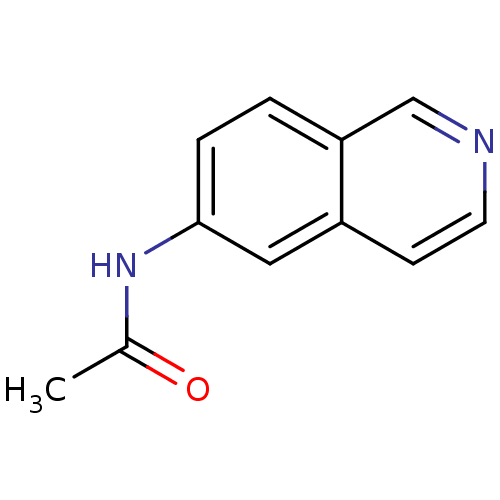 (CHEMBL285116 | N-Isoquinolin-6-yl-acetamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126267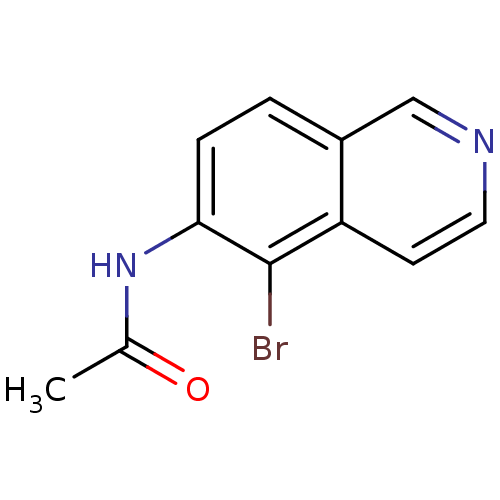 (CHEMBL29480 | N-(5-Bromo-isoquinolin-6-yl)-acetami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126266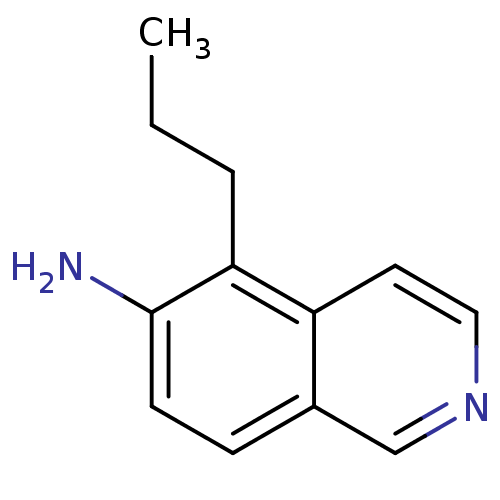 (5-Propyl-isoquinolin-6-ylamine | CHEMBL285794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126271 (1-Phthalazin-6-yl-3-m-tolyl-urea | CHEMBL27189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126264 (5-Methyl-isoquinolin-6-ylamine | CHEMBL284662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126274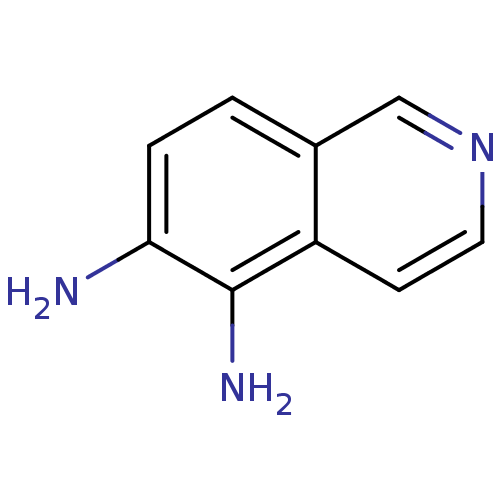 (CHEMBL27753 | Isoquinoline-5,6-diamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50126269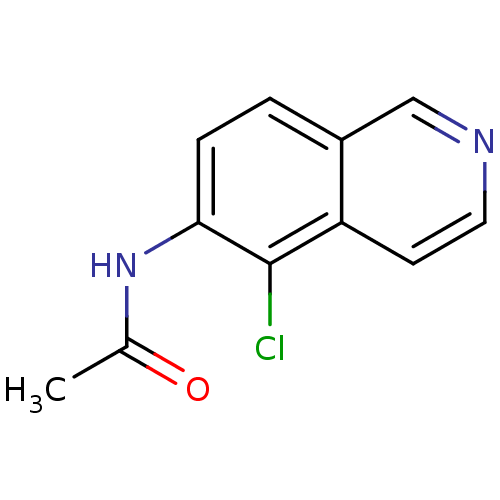 (CHEMBL282997 | N-(5-Chloro-isoquinolin-6-yl)-aceta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against inosine 5'-inosine monophosphate dehydrogenase type II (IMPDH II) | Bioorg Med Chem Lett 13: 1345-8 (2003) BindingDB Entry DOI: 10.7270/Q2CV4H3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||