 Found 20 hits of Enzyme Inhibition Constant Data
Found 20 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18864
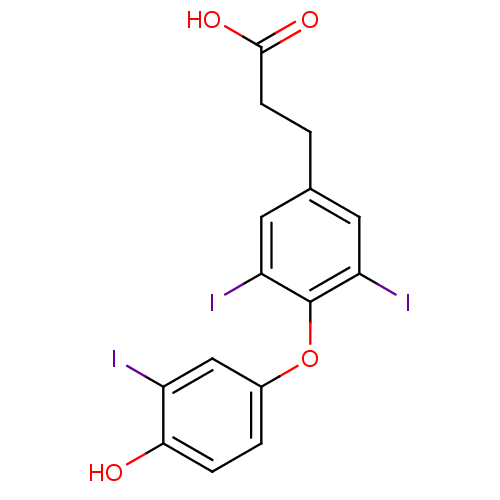
(3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...)Show InChI InChI=1S/C15H11I3O4/c16-10-7-9(2-3-13(10)19)22-15-11(17)5-8(6-12(15)18)1-4-14(20)21/h2-3,5-7,19H,1,4H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18865
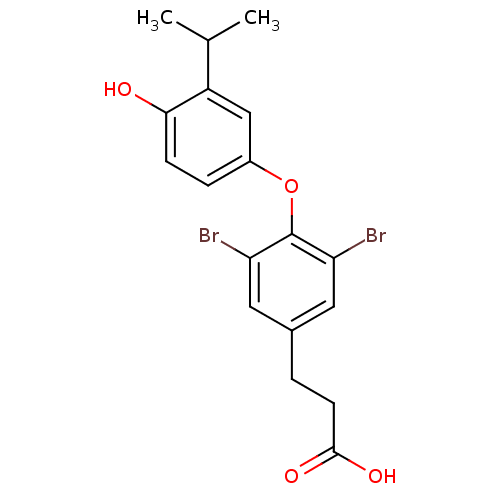
(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C18H18Br2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18864

(3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]pr...)Show InChI InChI=1S/C15H11I3O4/c16-10-7-9(2-3-13(10)19)22-15-11(17)5-8(6-12(15)18)1-4-14(20)21/h2-3,5-7,19H,1,4H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18862
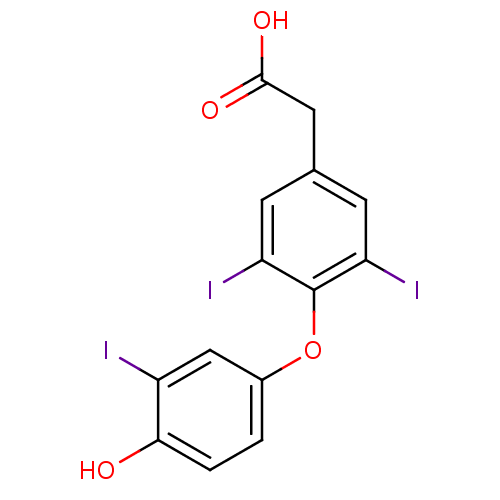
(2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...)Show InChI InChI=1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867
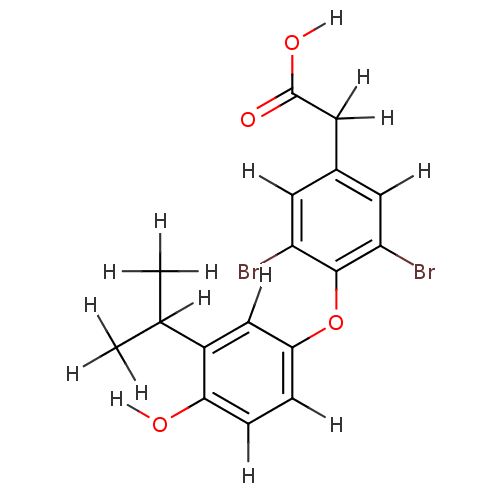
(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | 0.200 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18865

(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C18H18Br2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18863

((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...)Show SMILES CC(C)c1cc(Oc2c(I)cc(C[C@@H](N)C(O)=O)cc2I)ccc1O |r| Show InChI InChI=1S/C18H19I2NO4/c1-9(2)12-8-11(3-4-16(12)22)25-17-13(19)5-10(6-14(17)20)7-15(21)18(23)24/h3-6,8-9,15,22H,7,21H2,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18862

(2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...)Show InChI InChI=1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18863

((2R)-2-amino-3-{4-[4-hydroxy-3-(propan-2-yl)phenox...)Show SMILES CC(C)c1cc(Oc2c(I)cc(C[C@@H](N)C(O)=O)cc2I)ccc1O |r| Show InChI InChI=1S/C18H19I2NO4/c1-9(2)12-8-11(3-4-16(12)22)25-17-13(19)5-10(6-14(17)20)7-15(21)18(23)24/h3-6,8-9,15,22H,7,21H2,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18870
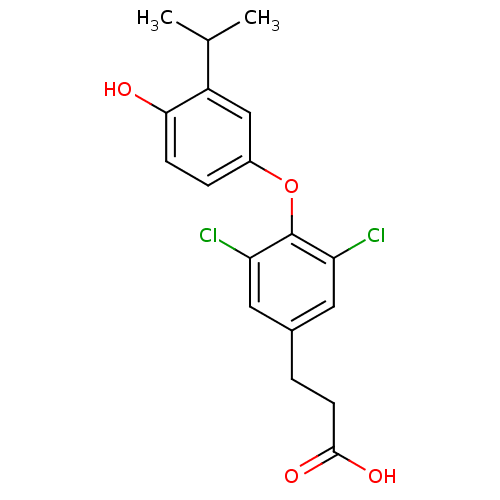
(3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.150 | n/a | 0.280 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860
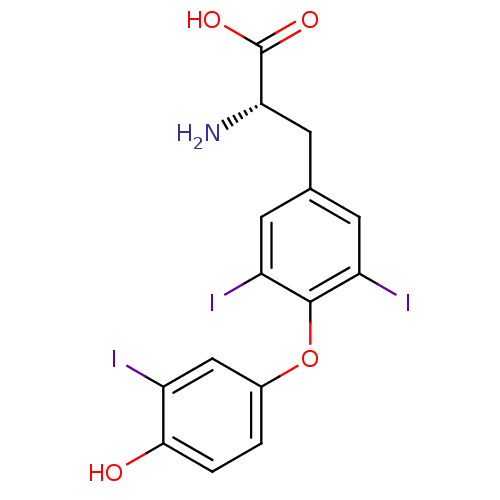
((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18870

(3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.760 | n/a | 0.300 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
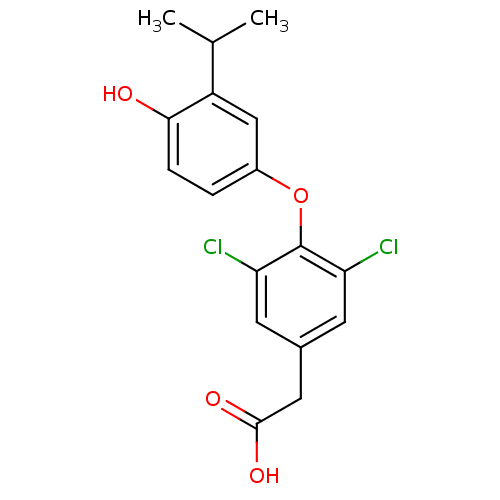
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | 3.5 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 0.380 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18866
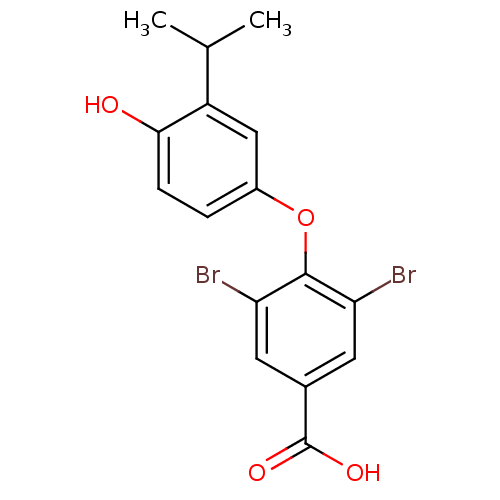
(3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy]be...)Show InChI InChI=1S/C16H14Br2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18866

(3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy]be...)Show InChI InChI=1S/C16H14Br2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18868
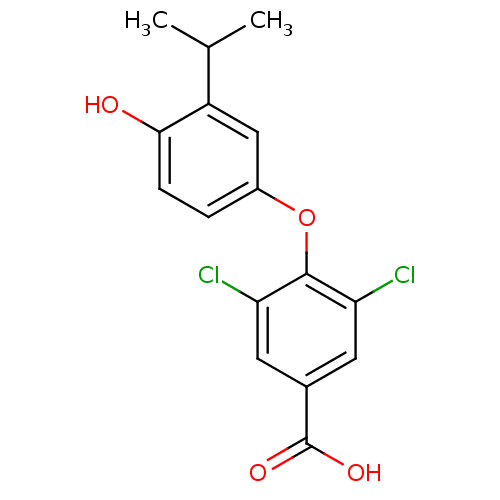
(3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenoxy]b...)Show InChI InChI=1S/C16H14Cl2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 21 | n/a | 150 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | 11 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18868

(3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenoxy]b...)Show InChI InChI=1S/C16H14Cl2O4/c1-8(2)11-7-10(3-4-14(11)19)22-15-12(17)5-9(16(20)21)6-13(15)18/h3-8,19H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 130 | n/a | 190 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data