

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50001950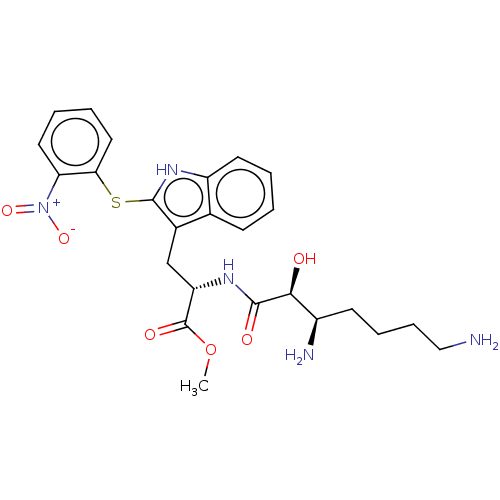 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain aminopeptidase | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50001947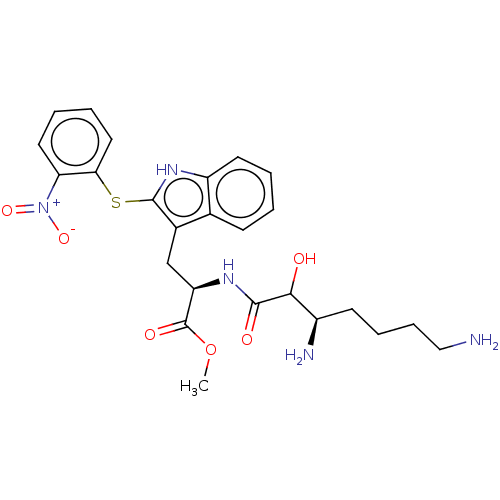 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain aminopeptidase | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50001941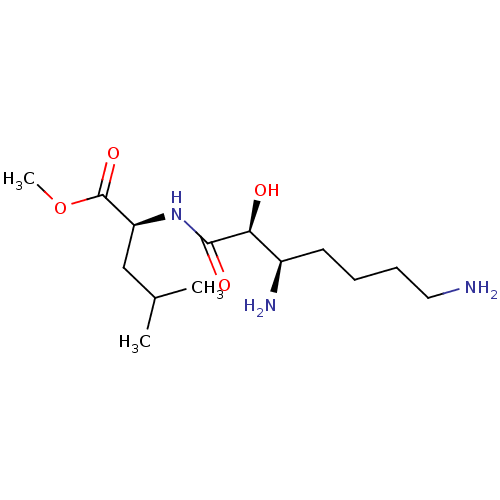 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-4-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001950 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as subst... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50368571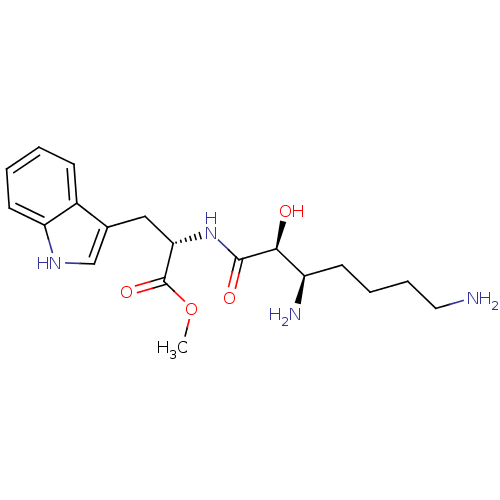 (CHEMBL1203858) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50001951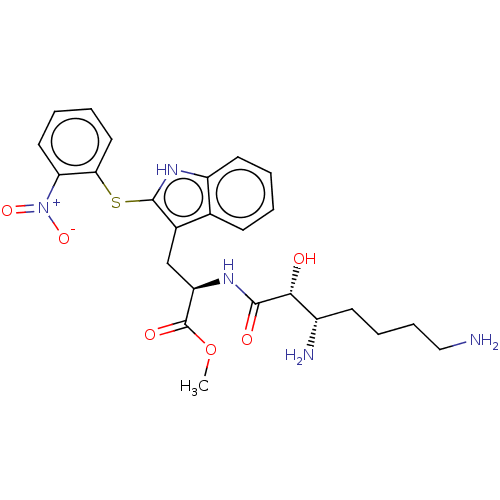 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain aminopeptidase | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50001944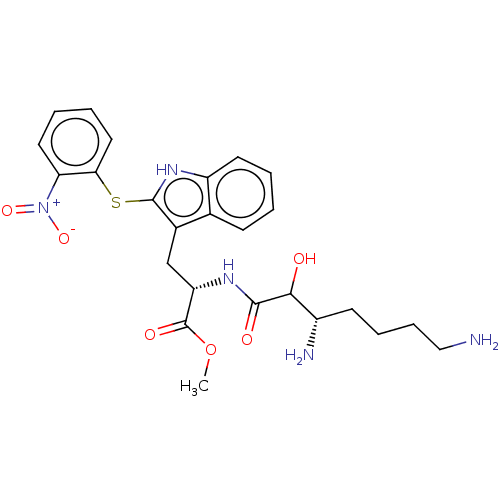 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibitory potency against purified membrane bound rat brain aminopeptidase | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001947 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as subst... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001950 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide ... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001941 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-4-methyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001951 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as subst... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001949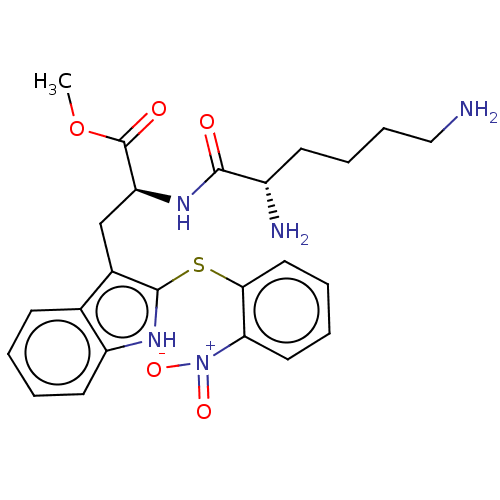 (2-(2,6-Diamino-hexanoylamino)-3-[2-(2-nitro-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001949 (2-(2,6-Diamino-hexanoylamino)-3-[2-(2-nitro-phenyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001941 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-4-methyl-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001947 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide ... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50368567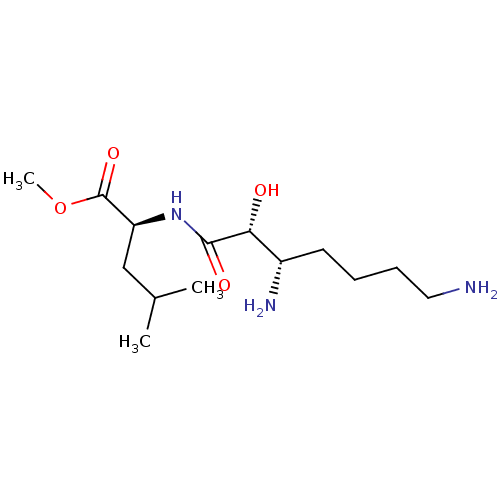 (CHEMBL1794905) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide ... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50368569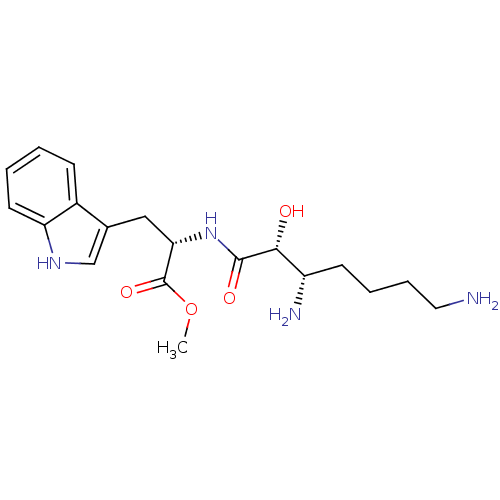 (CHEMBL1794904) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50368568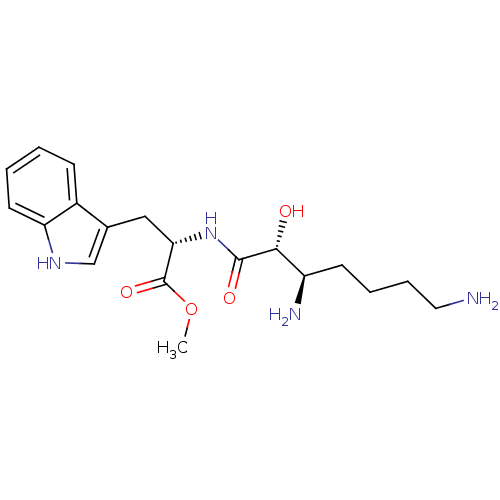 (CHEMBL1203859) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001944 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50368570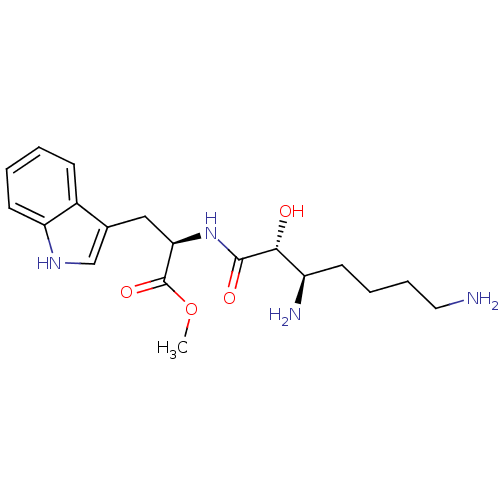 (CHEMBL1794908) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001944 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001951 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-[2-(2-n...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide ... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50368571 (CHEMBL1203858) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001945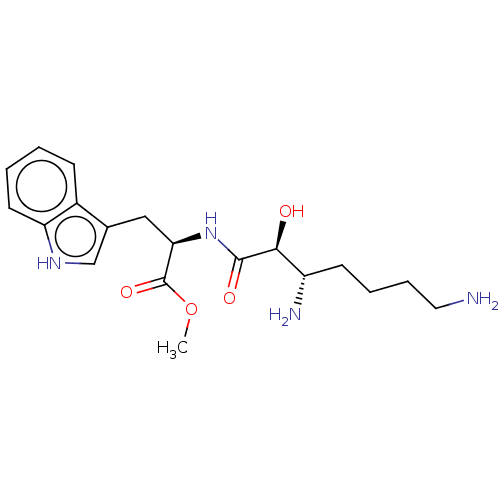 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-(1H-ind...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001946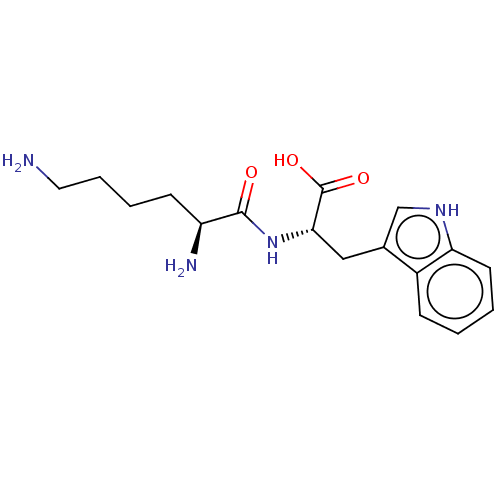 (2-(6-Amino-2-aminomethyl-hexanoylamino)-3-(1H-indo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as subst... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50368570 (CHEMBL1794908) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50368568 (CHEMBL1203859) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001948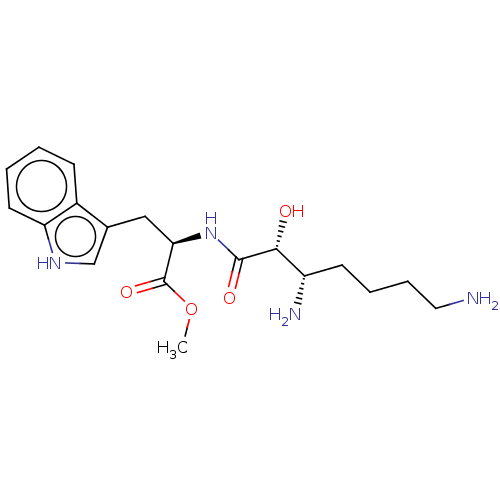 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-(1H-ind...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50001948 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-(1H-ind...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50368567 (CHEMBL1794905) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M (AP-M) using [3H]Leu-enkephalin as subst... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Rattus norvegicus) | BDBM50368569 (CHEMBL1794904) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase M using [3H]Leu-enkephalin as substrate | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001945 (2-(3,7-Diamino-2-hydroxy-heptanoylamino)-3-(1H-ind...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B using L-lysine-beta napthylamide as subs... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM50001946 (2-(6-Amino-2-aminomethyl-hexanoylamino)-3-(1H-indo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against purified membrane bound rat brain Aminopeptidase B (AP-B) using L-lysine-beta napthylamide ... | J Med Chem 35: 889-95 (1992) BindingDB Entry DOI: 10.7270/Q2X92BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||