

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004182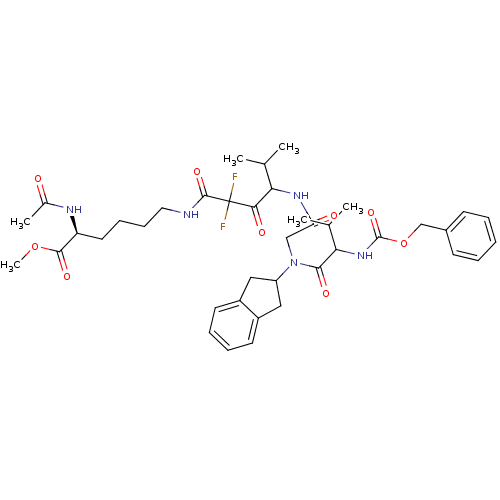 (2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004192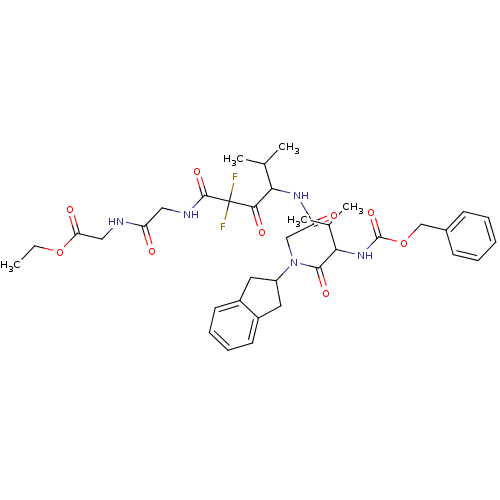 (CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184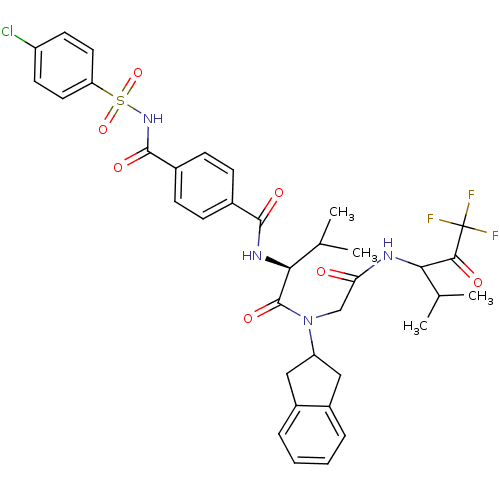 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004190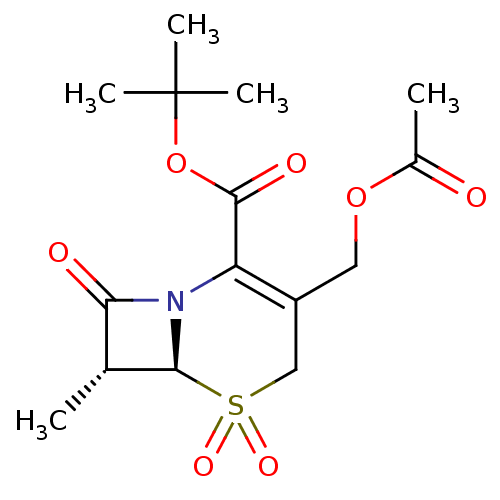 (3-Acetoxymethyl-7-methyl-5,5,8-trioxo-5lambda*6*-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187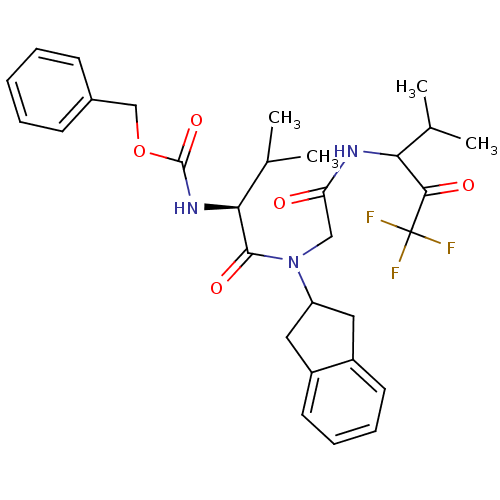 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004185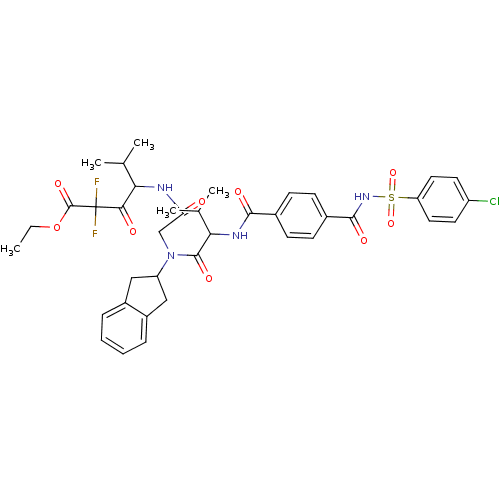 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004183 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004189 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004186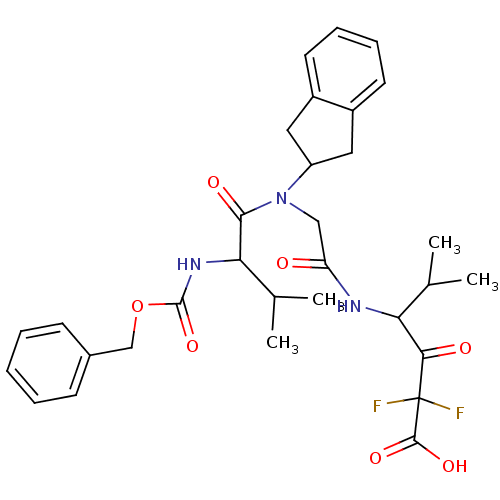 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004188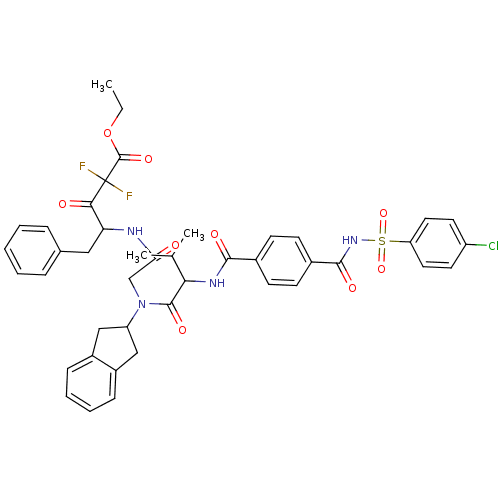 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004191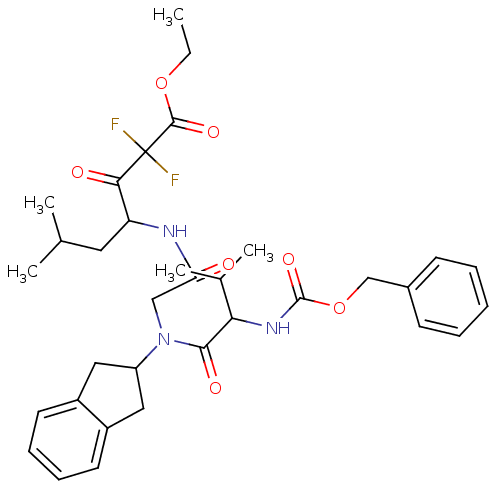 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||