 Found 94 hits of Enzyme Inhibition Constant Data
Found 94 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599
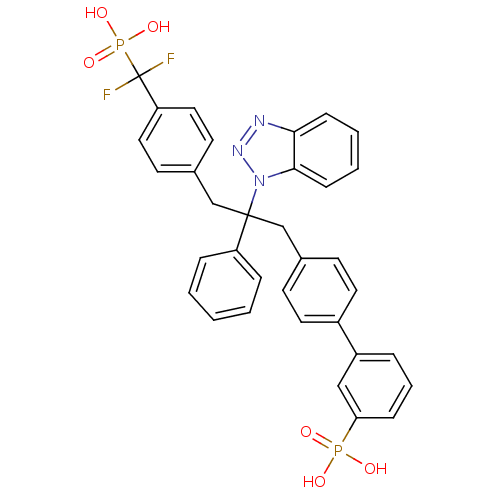
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142323
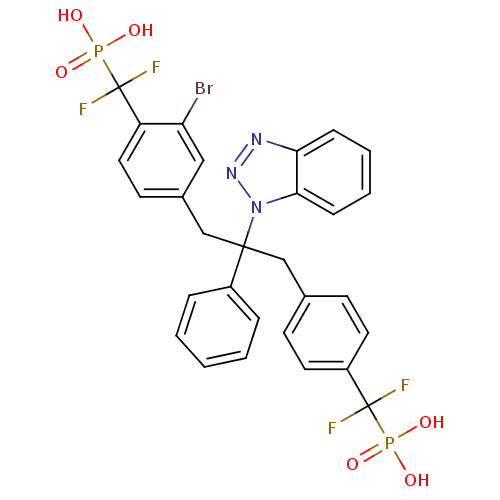
(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142323

(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13595
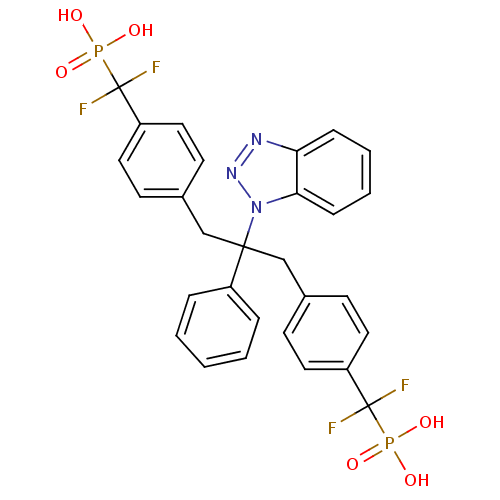
(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142335
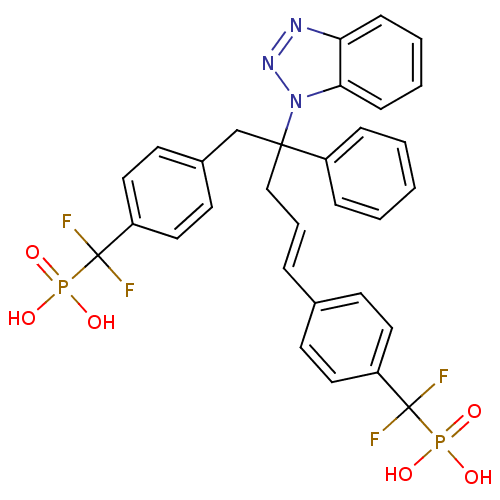
(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142328
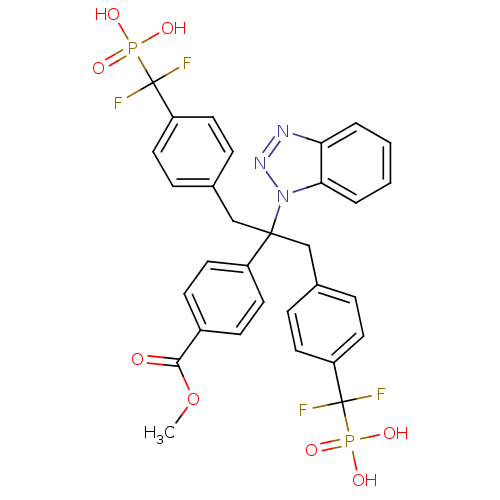
(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142324
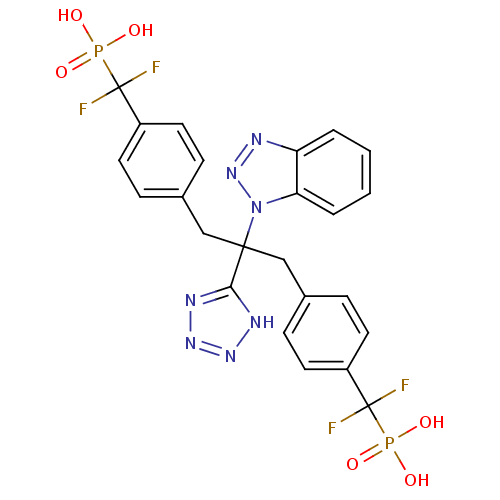
(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142335

(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142328

(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13595

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142329
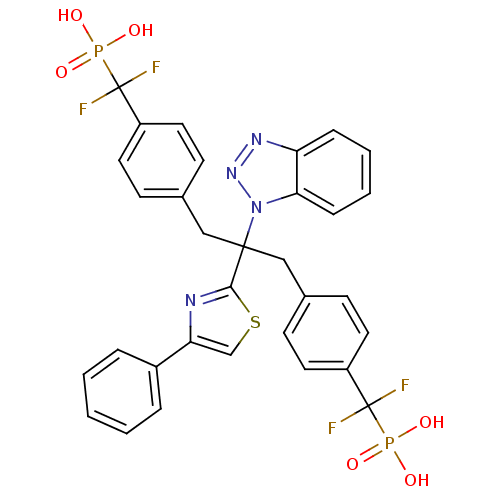
(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc(cs2)-c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2S/c33-31(34,47(41,42)43)24-14-10-21(11-15-24)18-30(40-28-9-5-4-8-26(28)38-39-40,29-37-27(20-49-29)23-6-2-1-3-7-23)19-22-12-16-25(17-13-22)32(35,36)48(44,45)46/h1-17,20H,18-19H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142330
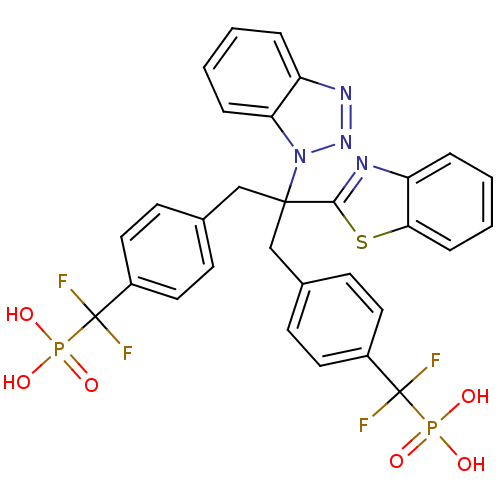
(CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc3ccccc3s2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H24F4N4O6P2S/c31-29(32,45(39,40)41)21-13-9-19(10-14-21)17-28(27-35-24-6-2-4-8-26(24)47-27,38-25-7-3-1-5-23(25)36-37-38)18-20-11-15-22(16-12-20)30(33,34)46(42,43)44/h1-16H,17-18H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142329

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc(cs2)-c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2S/c33-31(34,47(41,42)43)24-14-10-21(11-15-24)18-30(40-28-9-5-4-8-26(28)38-39-40,29-37-27(20-49-29)23-6-2-1-3-7-23)19-22-12-16-25(17-13-22)32(35,36)48(44,45)46/h1-17,20H,18-19H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142325
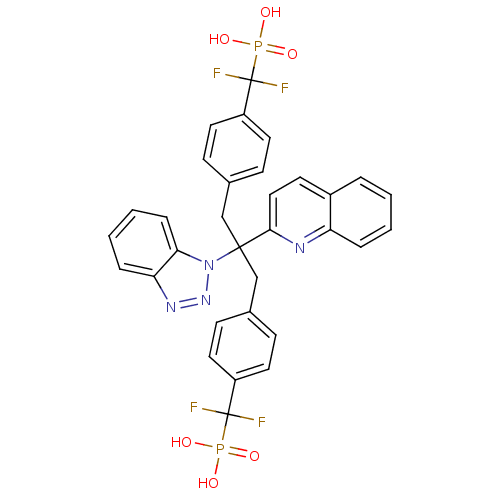
(CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc3ccccc3n2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2/c33-31(34,47(41,42)43)24-14-9-21(10-15-24)19-30(40-28-8-4-3-7-27(28)38-39-40,29-18-13-23-5-1-2-6-26(23)37-29)20-22-11-16-25(17-12-22)32(35,36)48(44,45)46/h1-18H,19-20H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142330

(CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc3ccccc3s2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H24F4N4O6P2S/c31-29(32,45(39,40)41)21-13-9-19(10-14-21)17-28(27-35-24-6-2-4-8-26(24)47-27,38-25-7-3-1-5-23(25)36-37-38)18-20-11-15-22(16-12-20)30(33,34)46(42,43)44/h1-16H,17-18H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13598
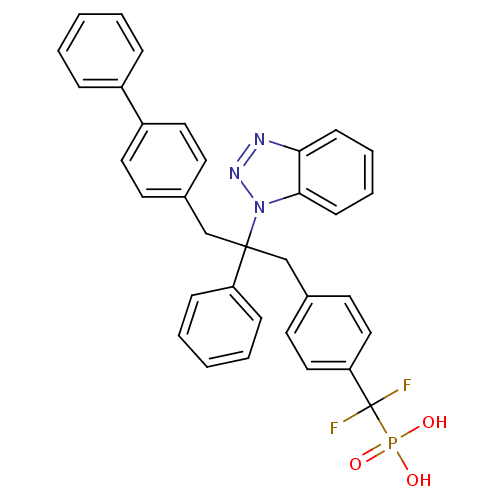
(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142325

(CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc3ccccc3n2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2/c33-31(34,47(41,42)43)24-14-9-21(10-15-24)19-30(40-28-8-4-3-7-27(28)38-39-40,29-18-13-23-5-1-2-6-26(23)37-29)20-22-11-16-25(17-12-22)32(35,36)48(44,45)46/h1-18H,19-20H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142324

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13598

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142333

(CHEMBL9321 | {[4-(2-Benzotriazol-1-yl-2,5-diphenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(CCCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H28F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-8,10-11,13-20H,9,12,21-22H2,(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13815
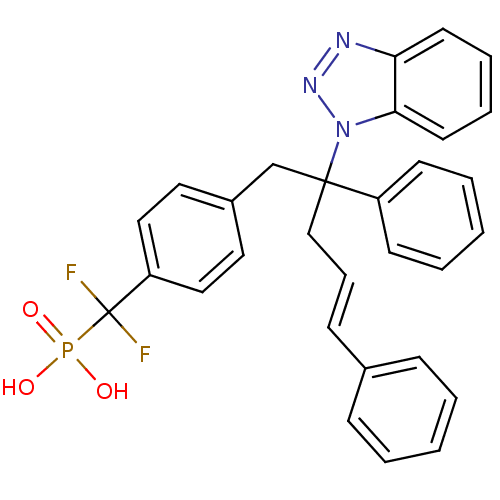
(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2,5-diphe...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H26F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-20H,21-22H2,(H2,36,37,38)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13815

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2,5-diphe...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H26F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-20H,21-22H2,(H2,36,37,38)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142325

(CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc3ccccc3n2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2/c33-31(34,47(41,42)43)24-14-9-21(10-15-24)19-30(40-28-8-4-3-7-27(28)38-39-40,29-18-13-23-5-1-2-6-26(23)37-29)20-22-11-16-25(17-12-22)32(35,36)48(44,45)46/h1-18H,19-20H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142334

(({4-[2-Benzotriazol-1-yl-3-(4-benzyloxy-phenyl)-2-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(OCc3ccccc3)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H30F2N3O4P/c36-35(37,45(41,42)43)30-19-15-26(16-20-30)23-34(29-11-5-2-6-12-29,40-33-14-8-7-13-32(33)38-39-40)24-27-17-21-31(22-18-27)44-25-28-9-3-1-4-10-28/h1-22H,23-25H2,(H2,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142322

(({4-[2-Benzotriazol-1-yl-2-phenyl-3-(2'-sulfamoyl-...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N4O5PS/c35-34(36,46(41,42)43)28-20-16-25(17-21-28)23-33(27-8-2-1-3-9-27,40-31-12-6-5-11-30(31)38-39-40)22-24-14-18-26(19-15-24)29-10-4-7-13-32(29)47(37,44)45/h1-21H,22-23H2,(H2,37,44,45)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142333

(CHEMBL9321 | {[4-(2-Benzotriazol-1-yl-2,5-diphenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(CCCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H28F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-8,10-11,13-20H,9,12,21-22H2,(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142326

(CHEMBL276648 | [(4-{2-Benzotriazol-1-yl-2-phenyl-1...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)C(C(c1ccccc1)n1nnc2ccccc12)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H26F2N7O3P/c35-34(36,47(44,45)46)26-20-18-24(19-21-26)31(32(25-8-2-1-3-9-25)43-30-13-7-6-12-29(30)37-42-43)23-16-14-22(15-17-23)27-10-4-5-11-28(27)33-38-40-41-39-33/h1-21,31-32H,(H2,44,45,46)(H,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142322

(({4-[2-Benzotriazol-1-yl-2-phenyl-3-(2'-sulfamoyl-...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N4O5PS/c35-34(36,46(41,42)43)28-20-16-25(17-21-28)23-33(27-8-2-1-3-9-27,40-31-12-6-5-11-30(31)38-39-40)22-24-14-18-26(19-15-24)29-10-4-7-13-32(29)47(37,44)45/h1-21H,22-23H2,(H2,37,44,45)(H2,41,42,43) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142328

(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142326

(CHEMBL276648 | [(4-{2-Benzotriazol-1-yl-2-phenyl-1...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)C(C(c1ccccc1)n1nnc2ccccc12)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H26F2N7O3P/c35-34(36,47(44,45)46)26-20-18-24(19-21-26)31(32(25-8-2-1-3-9-25)43-30-13-7-6-12-29(30)37-42-43)23-16-14-22(15-17-23)27-10-4-5-11-28(27)33-38-40-41-39-33/h1-21,31-32H,(H2,44,45,46)(H,38,39,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142330

(CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc3ccccc3s2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H24F4N4O6P2S/c31-29(32,45(39,40)41)21-13-9-19(10-14-21)17-28(27-35-24-6-2-4-8-26(24)47-27,38-25-7-3-1-5-23(25)36-37-38)18-20-11-15-22(16-12-20)30(33,34)46(42,43)44/h1-16H,17-18H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142331

(({4-[2-Benzotriazol-1-yl-3-(4-bromo-phenyl)-2-phen...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(Br)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C28H23BrF2N3O3P/c29-24-16-12-21(13-17-24)19-27(22-6-2-1-3-7-22,34-26-9-5-4-8-25(26)32-33-34)18-20-10-14-23(15-11-20)28(30,31)38(35,36)37/h1-17H,18-19H2,(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142331

(({4-[2-Benzotriazol-1-yl-3-(4-bromo-phenyl)-2-phen...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(Br)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C28H23BrF2N3O3P/c29-24-16-12-21(13-17-24)19-27(22-6-2-1-3-7-22,34-26-9-5-4-8-25(26)32-33-34)18-20-10-14-23(15-11-20)28(30,31)38(35,36)37/h1-17H,18-19H2,(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142334

(({4-[2-Benzotriazol-1-yl-3-(4-benzyloxy-phenyl)-2-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(OCc3ccccc3)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H30F2N3O4P/c36-35(37,45(41,42)43)30-19-15-26(16-20-30)23-34(29-11-5-2-6-12-29,40-33-14-8-7-13-32(33)38-39-40)24-27-17-21-31(22-18-27)44-25-28-9-3-1-4-10-28/h1-22H,23-25H2,(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142335

(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13595

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142323

(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142329

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc(cs2)-c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2S/c33-31(34,47(41,42)43)24-14-10-21(11-15-24)18-30(40-28-9-5-4-8-26(28)38-39-40,29-37-27(20-49-29)23-6-2-1-3-7-23)19-22-12-16-25(17-13-22)32(35,36)48(44,45)46/h1-17,20H,18-19H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142327

(2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)OCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24F2N3O5P/c30-29(31,40(36,37)38)24-17-15-21(16-18-24)19-28(23-11-5-2-6-12-23,27(35)39-20-22-9-3-1-4-10-22)34-26-14-8-7-13-25(26)32-33-34/h1-18H,19-20H2,(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142327

(2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)OCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24F2N3O5P/c30-29(31,40(36,37)38)24-17-15-21(16-18-24)19-28(23-11-5-2-6-12-23,27(35)39-20-22-9-3-1-4-10-22)34-26-14-8-7-13-25(26)32-33-34/h1-18H,19-20H2,(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142326

(CHEMBL276648 | [(4-{2-Benzotriazol-1-yl-2-phenyl-1...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)C(C(c1ccccc1)n1nnc2ccccc12)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H26F2N7O3P/c35-34(36,47(44,45)46)26-20-18-24(19-21-26)31(32(25-8-2-1-3-9-25)43-30-13-7-6-12-29(30)37-42-43)23-16-14-22(15-17-23)27-10-4-5-11-28(27)33-38-40-41-39-33/h1-21,31-32H,(H2,44,45,46)(H,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13815

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2,5-diphe...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H26F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-20H,21-22H2,(H2,36,37,38)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142333

(CHEMBL9321 | {[4-(2-Benzotriazol-1-yl-2,5-diphenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(CCCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H28F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-8,10-11,13-20H,9,12,21-22H2,(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142322

(({4-[2-Benzotriazol-1-yl-2-phenyl-3-(2'-sulfamoyl-...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N4O5PS/c35-34(36,46(41,42)43)28-20-16-25(17-21-28)23-33(27-8-2-1-3-9-27,40-31-12-6-5-11-30(31)38-39-40)22-24-14-18-26(19-15-24)29-10-4-7-13-32(29)47(37,44)45/h1-21H,22-23H2,(H2,37,44,45)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142324

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13598

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142331

(({4-[2-Benzotriazol-1-yl-3-(4-bromo-phenyl)-2-phen...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(Br)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C28H23BrF2N3O3P/c29-24-16-12-21(13-17-24)19-27(22-6-2-1-3-7-22,34-26-9-5-4-8-25(26)32-33-34)18-20-10-14-23(15-11-20)28(30,31)38(35,36)37/h1-17H,18-19H2,(H2,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142327

(2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)OCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24F2N3O5P/c30-29(31,40(36,37)38)24-17-15-21(16-18-24)19-28(23-11-5-2-6-12-23,27(35)39-20-22-9-3-1-4-10-22)34-26-14-8-7-13-25(26)32-33-34/h1-18H,19-20H2,(H2,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142334

(({4-[2-Benzotriazol-1-yl-3-(4-benzyloxy-phenyl)-2-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(OCc3ccccc3)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H30F2N3O4P/c36-35(37,45(41,42)43)30-19-15-26(16-20-30)23-34(29-11-5-2-6-12-29,40-33-14-8-7-13-32(33)38-39-40)24-27-17-21-31(22-18-27)44-25-28-9-3-1-4-10-28/h1-22H,23-25H2,(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142324

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nnn[nH]2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C24H21F4N7O6P2/c25-23(26,42(36,37)38)17-9-5-15(6-10-17)13-22(21-30-32-33-31-21,35-20-4-2-1-3-19(20)29-34-35)14-16-7-11-18(12-8-16)24(27,28)43(39,40)41/h1-12H,13-14H2,(H2,36,37,38)(H2,39,40,41)(H,30,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50142332

(CHEMBL9363 | {[4-(2-Benzotriazol-1-yl-2-phenyl-eth...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C21H18F2N3O3P/c22-21(23,30(27,28)29)17-12-10-15(11-13-17)14-20(16-6-2-1-3-7-16)26-19-9-5-4-8-18(19)24-25-26/h1-13,20H,14H2,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against T cell protein tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142332

(CHEMBL9363 | {[4-(2-Benzotriazol-1-yl-2-phenyl-eth...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C21H18F2N3O3P/c22-21(23,30(27,28)29)17-12-10-15(11-13-17)14-20(16-6-2-1-3-7-16)26-19-9-5-4-8-18(19)24-25-26/h1-13,20H,14H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142330

(CHEMBL8662 | [(4-{2-Benzothiazol-2-yl-2-benzotriaz...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc3ccccc3s2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H24F4N4O6P2S/c31-29(32,45(39,40)41)21-13-9-19(10-14-21)17-28(27-35-24-6-2-4-8-26(24)47-27,38-25-7-3-1-5-23(25)36-37-38)18-20-11-15-22(16-12-20)30(33,34)46(42,43)44/h1-16H,17-18H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142331

(({4-[2-Benzotriazol-1-yl-3-(4-bromo-phenyl)-2-phen...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(Br)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C28H23BrF2N3O3P/c29-24-16-12-21(13-17-24)19-27(22-6-2-1-3-7-22,34-26-9-5-4-8-25(26)32-33-34)18-20-10-14-23(15-11-20)28(30,31)38(35,36)37/h1-17H,18-19H2,(H2,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142323

(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142333

(CHEMBL9321 | {[4-(2-Benzotriazol-1-yl-2,5-diphenyl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(CCCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H28F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-8,10-11,13-20H,9,12,21-22H2,(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142325

(CHEMBL273474 | [(4-{2-Benzotriazol-1-yl-3-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc3ccccc3n2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2/c33-31(34,47(41,42)43)24-14-9-21(10-15-24)19-30(40-28-8-4-3-7-27(28)38-39-40,29-18-13-23-5-1-2-6-26(23)37-29)20-22-11-16-25(17-12-22)32(35,36)48(44,45)46/h1-18H,19-20H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13596

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccc(F)c(F)c2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H23F6N3O6P2/c30-23-14-13-22(15-24(23)31)27(38-26-4-2-1-3-25(26)36-37-38,16-18-5-9-20(10-6-18)28(32,33)45(39,40)41)17-19-7-11-21(12-8-19)29(34,35)46(42,43)44/h1-15H,16-17H2,(H2,39,40,41)(H2,42,43,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142328

(4-{1-Benzotriazol-1-yl-1-[4-(difluoro-phosphono-me...)Show SMILES COC(=O)c1ccc(cc1)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)n1nnc2ccccc12 Show InChI InChI=1S/C31H27F4N3O8P2/c1-46-28(39)22-10-16-23(17-11-22)29(38-27-5-3-2-4-26(27)36-37-38,18-20-6-12-24(13-7-20)30(32,33)47(40,41)42)19-21-8-14-25(15-9-21)31(34,35)48(43,44)45/h2-17H,18-19H2,1H3,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142335

(CHEMBL274435 | [(4-{4-Benzotriazol-1-yl-5-[4-(difl...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C31H27F4N3O6P2/c32-30(33,45(39,40)41)25-16-12-22(13-17-25)7-6-20-29(24-8-2-1-3-9-24,38-28-11-5-4-10-27(28)36-37-38)21-23-14-18-26(19-15-23)31(34,35)46(42,43)44/h1-19H,20-21H2,(H2,39,40,41)(H2,42,43,44)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142322

(({4-[2-Benzotriazol-1-yl-2-phenyl-3-(2'-sulfamoyl-...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N4O5PS/c35-34(36,46(41,42)43)28-20-16-25(17-21-28)23-33(27-8-2-1-3-9-27,40-31-12-6-5-11-30(31)38-39-40)22-24-14-18-26(19-15-24)29-10-4-7-13-32(29)47(37,44)45/h1-21H,22-23H2,(H2,37,44,45)(H2,41,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13602

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES Cc1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C38H32F2N4O6P2/c1-25-11-16-29-21-30(22-35(36(29)41-25)51(45,46)47)28-17-12-26(13-18-28)23-37(31-7-3-2-4-8-31,44-34-10-6-5-9-33(34)42-43-44)24-27-14-19-32(20-15-27)38(39,40)52(48,49)50/h2-22H,23-24H2,1H3,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142326

(CHEMBL276648 | [(4-{2-Benzotriazol-1-yl-2-phenyl-1...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)C(C(c1ccccc1)n1nnc2ccccc12)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H26F2N7O3P/c35-34(36,47(44,45)46)26-20-18-24(19-21-26)31(32(25-8-2-1-3-9-25)43-30-13-7-6-12-29(30)37-42-43)23-16-14-22(15-17-23)27-10-4-5-11-28(27)33-38-40-41-39-33/h1-21,31-32H,(H2,44,45,46)(H,38,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13595

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluoro...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H25F4N3O6P2/c30-28(31,43(37,38)39)23-14-10-20(11-15-23)18-27(22-6-2-1-3-7-22,36-26-9-5-4-8-25(26)34-35-36)19-21-12-16-24(17-13-21)29(32,33)44(40,41)42/h1-17H,18-19H2,(H2,37,38,39)(H2,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142327

(2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)OCc2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24F2N3O5P/c30-29(31,40(36,37)38)24-17-15-21(16-18-24)19-28(23-11-5-2-6-12-23,27(35)39-20-22-9-3-1-4-10-22)34-26-14-8-7-13-25(26)32-33-34/h1-18H,19-20H2,(H2,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142332

(CHEMBL9363 | {[4-(2-Benzotriazol-1-yl-2-phenyl-eth...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C21H18F2N3O3P/c22-21(23,30(27,28)29)17-12-10-15(11-13-17)14-20(16-6-2-1-3-7-16)26-19-9-5-4-8-18(19)24-25-26/h1-13,20H,14H2,(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13815

(({4-[(4E)-2-(1H-1,2,3-benzotriazol-1-yl)-2,5-diphe...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C\C=C\c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C30H26F2N3O3P/c31-30(32,39(36,37)38)26-19-17-24(18-20-26)22-29(25-13-5-2-6-14-25,21-9-12-23-10-3-1-4-11-23)35-28-16-8-7-15-27(28)33-34-35/h1-20H,21-22H2,(H2,36,37,38)/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein tyrosine phosphatase 1B (PTP1B) overexpressed in intact Sf9 cell assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142329

(({4-[2-Benzotriazol-1-yl-3-[4-(difluoro-phosphono-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2nc(cs2)-c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C32H26F4N4O6P2S/c33-31(34,47(41,42)43)24-14-10-21(11-15-24)18-30(40-28-9-5-4-8-26(28)38-39-40,29-37-27(20-49-29)23-6-2-1-3-7-23)19-22-12-16-25(17-13-22)32(35,36)48(44,45)46/h1-17,20H,18-19H2,(H2,41,42,43)(H2,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50142334

(({4-[2-Benzotriazol-1-yl-3-(4-benzyloxy-phenyl)-2-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(OCc3ccccc3)cc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C35H30F2N3O4P/c36-35(37,45(41,42)43)30-19-15-26(16-20-30)23-34(29-11-5-2-6-12-29,40-33-14-8-7-13-32(33)38-39-40)24-27-17-21-31(22-18-27)44-25-28-9-3-1-4-10-28/h1-22H,23-25H2,(H2,41,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM13598

(({4-[2-(1H-1,2,3-benzotriazol-1-yl)-2-phenyl-3-(4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)-c2ccccc2)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H28F2N3O3P/c35-34(36,43(40,41)42)30-21-17-26(18-22-30)24-33(29-11-5-2-6-12-29,39-32-14-8-7-13-31(32)37-38-39)23-25-15-19-28(20-16-25)27-9-3-1-4-10-27/h1-22H,23-24H2,(H2,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 protein-tyrosine phosphatase |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data