

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM9475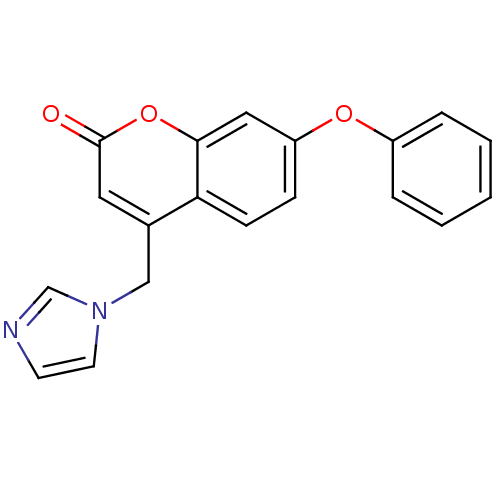 (4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9465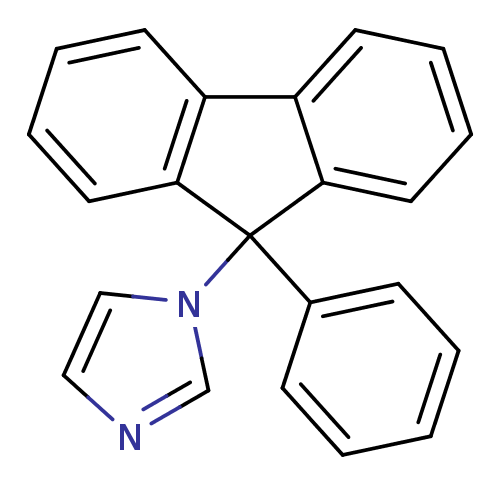 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9483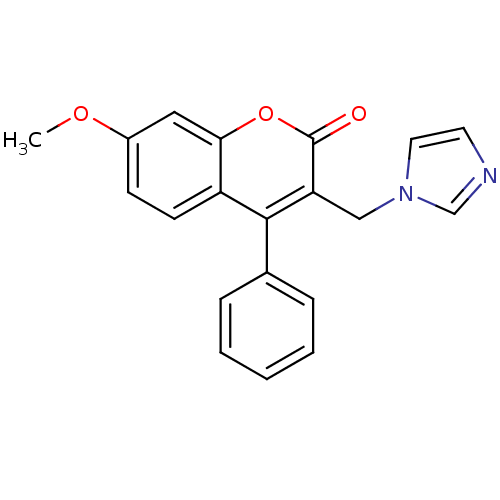 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-4-phenyl-2H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9477 (6-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9485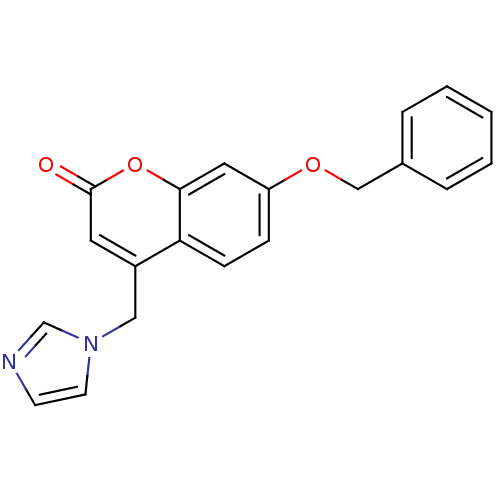 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9476 (5-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9471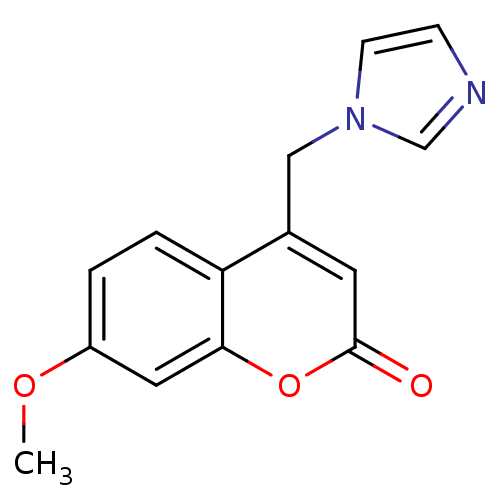 (4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9478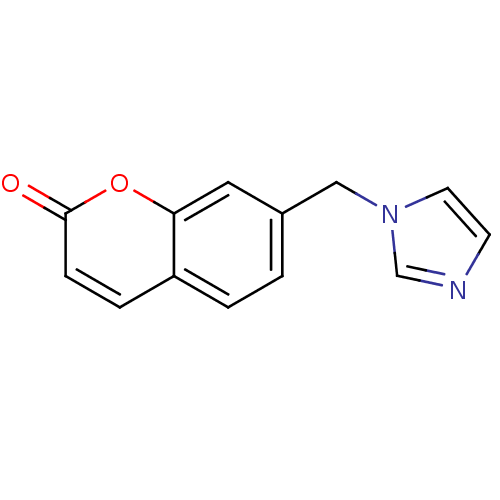 (7-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9473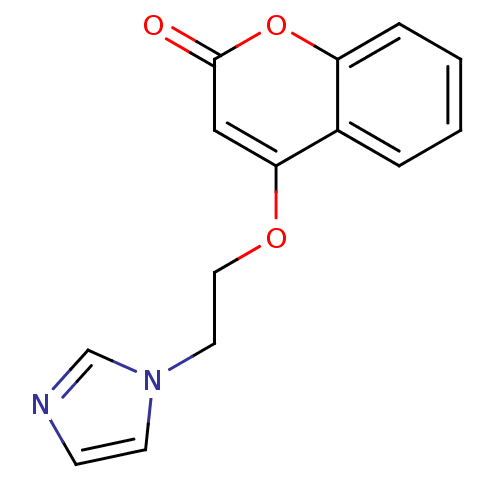 (4-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9486 (6-(1H-Imidazol-1-ylmethyl)-2H-chrome-2-thione | 6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9470 (4-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9469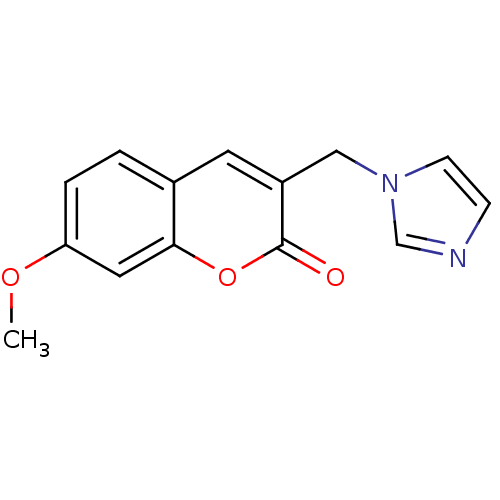 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9462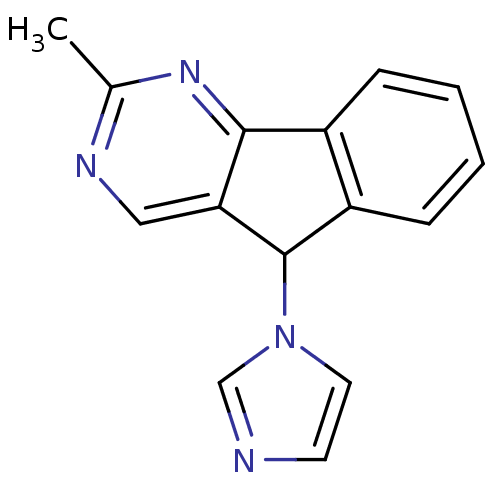 (1-{2-methyl-5H-indeno[1,2-d]pyrimidin-5-yl}-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9464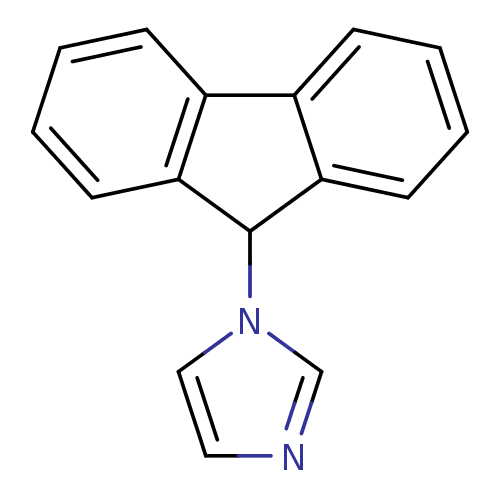 (1-(9H-Fluoren-9-yl)-1H-imidazole | CHEMBL225447 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9472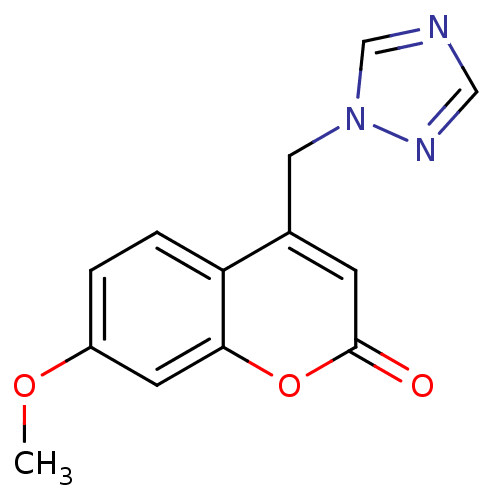 (7-Methoxy-4-(1H-1,2,4-triazol-1-ylmethyl)-2H-chrom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9468 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,4-triazole | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9482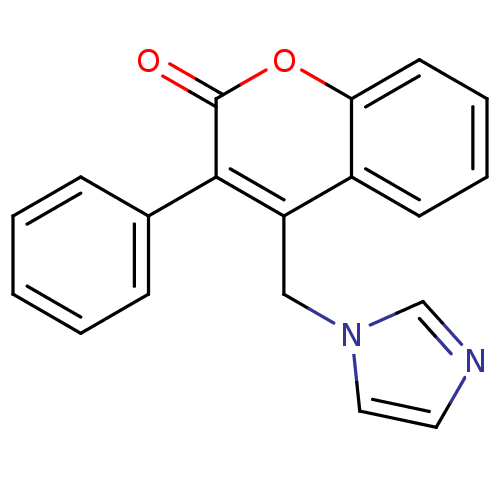 (4-(1H-Imidazol-1-ylmethyl)-3-phenyl-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8935 (5-[(6-methoxy-1-methyl-3,4-dihydronaphthalen-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9463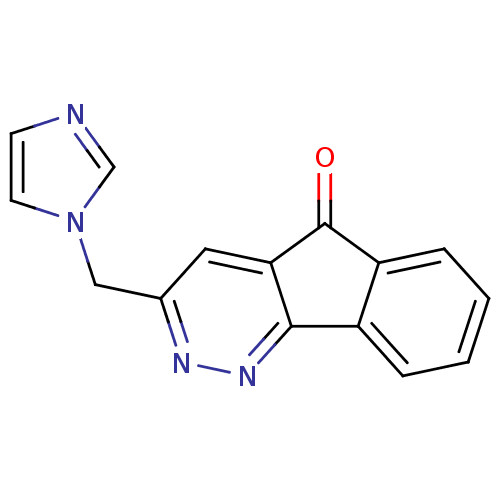 (3-(1H-Imidazol-1-ylmethyl)-5H-indeno[1,2-c]pyridaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9480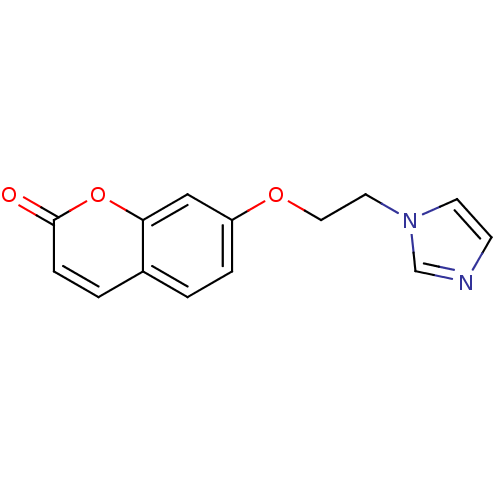 (7-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9481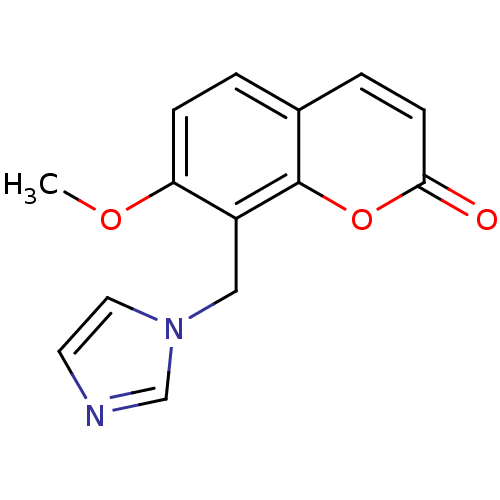 (8-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9483 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-4-phenyl-2H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9484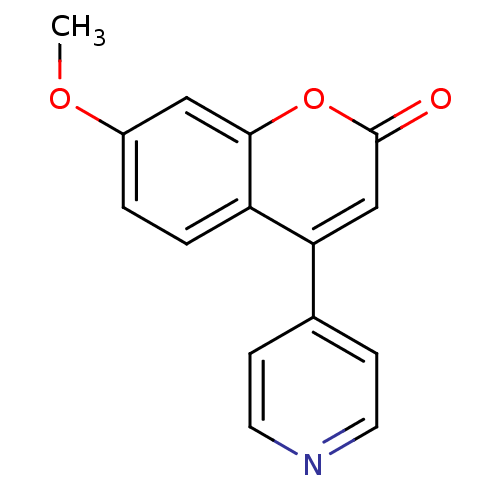 (7-methoxy-4-(pyridin-4-yl)-2H-chromen-2-one | Coum...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9485 (7-(benzyloxy)-4-(1H-imidazol-1-ylmethyl)-2H-chrome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9463 (3-(1H-Imidazol-1-ylmethyl)-5H-indeno[1,2-c]pyridaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9475 (4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9467 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,3-triazole | F...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9465 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9476 (5-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9473 (4-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9472 (7-Methoxy-4-(1H-1,2,4-triazol-1-ylmethyl)-2H-chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9470 (4-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9481 (8-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9480 (7-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9479 (7-(1H-1,2,4-Triazol-1-ylmethyl)-2H-chromen-2-one |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9469 (3-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9468 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,4-triazole | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9462 (1-{2-methyl-5H-indeno[1,2-d]pyrimidin-5-yl}-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9479 (7-(1H-1,2,4-Triazol-1-ylmethyl)-2H-chromen-2-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9482 (4-(1H-Imidazol-1-ylmethyl)-3-phenyl-2H-chromen-2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9477 (6-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9474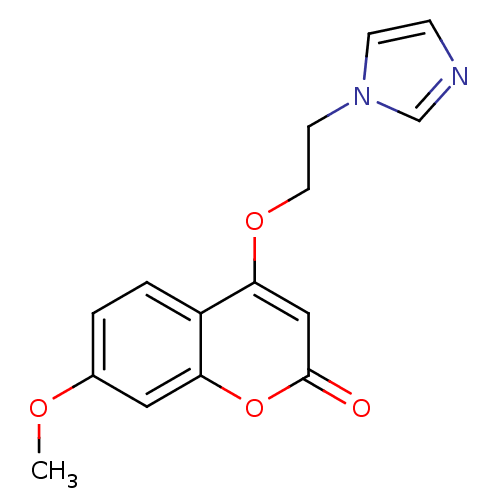 (4-[2-(1H-Imidazol-1-yl)ethoxy]-7-methoxy-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9486 (6-(1H-Imidazol-1-ylmethyl)-2H-chrome-2-thione | 6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9471 (4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9484 (7-methoxy-4-(pyridin-4-yl)-2H-chromen-2-one | Coum...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9466 (4-(9-Phenyl-9H-fluoren-9-yl)-4H-1,2,4-triazole | F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9466 (4-(9-Phenyl-9H-fluoren-9-yl)-4H-1,2,4-triazole | F...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9467 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,3-triazole | F...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM9478 (7-(1H-Imidazol-1-ylmethyl)-2H-chromen-2-one | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||