

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50003896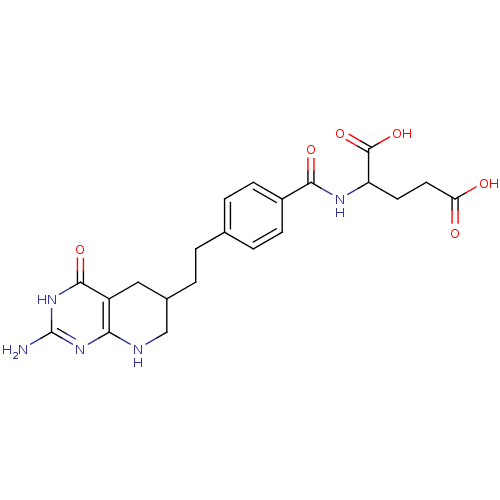 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50422000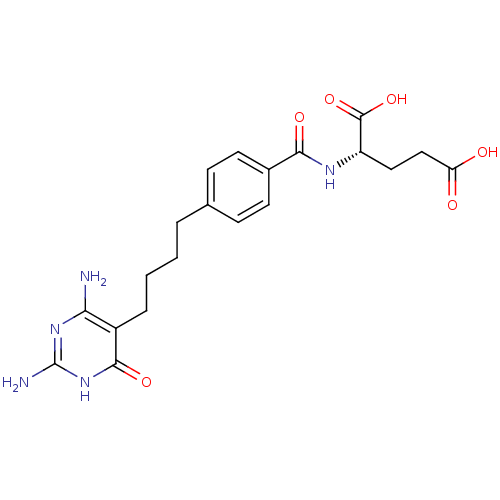 (DIDEAZAACYCLOTETRAHYDROFOLIC ACID) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005733 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005520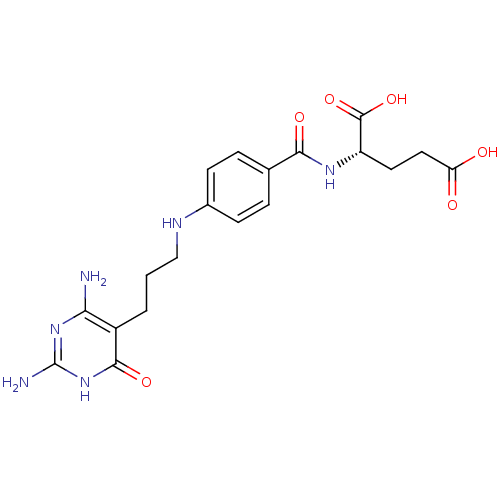 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005731 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005735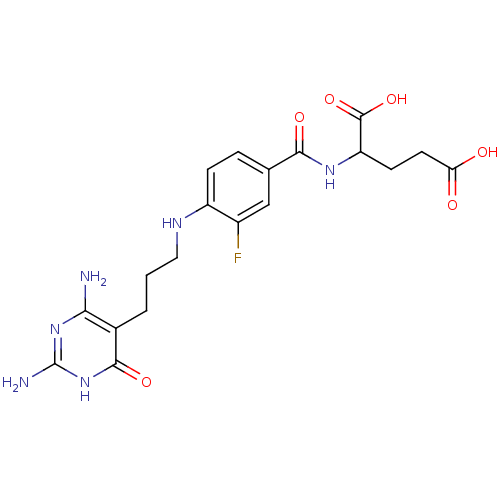 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005737 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005728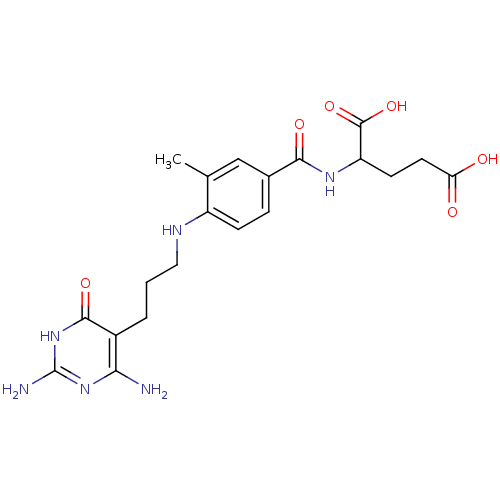 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005736 (2-{2-Chloro-4-[3-(2,4-diamino-6-oxo-1,6-dihydro-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005729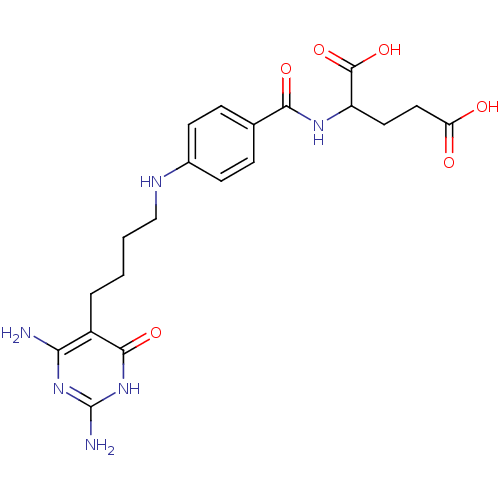 (2-{4-[4-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005734 (2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005739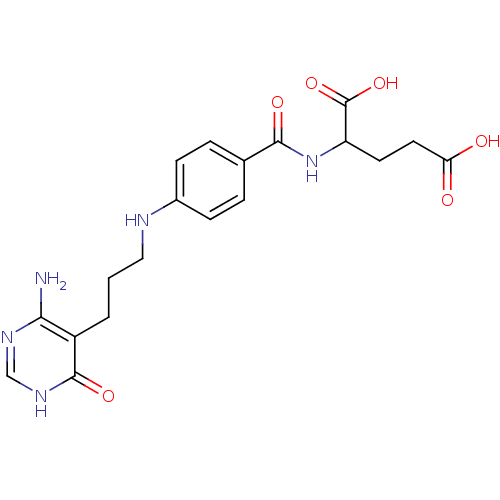 (2-{4-[3-(4-Amino-6-oxo-1,6-dihydro-pyrimidin-5-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50005520 ((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against MOLT-4 T-cell leukemia cell AICAR transformylase | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50005738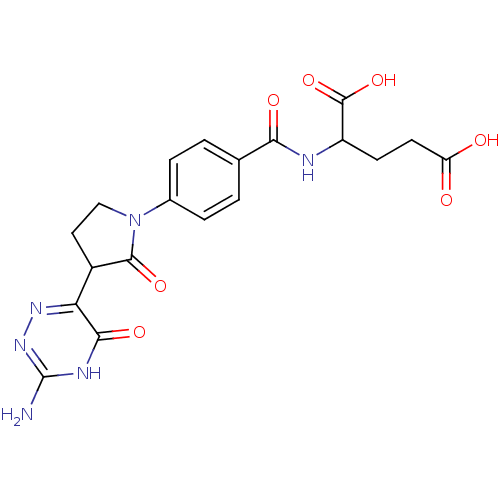 (2-{4-[3-(3-Amino-5-oxo-2,5-dihydro-[1,2,4]triazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against MOLT-4 T-cell leukemia cell AICAR transformylase | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||