 Found 37 hits of Enzyme Inhibition Constant Data
Found 37 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50240981
(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173405
((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K from humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173405

((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173404

((S)-2-[1-(4-Bromo-phenyl)-2,2,3,3,3-pentafluoro-pr...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C17H19BrF5N3O/c1-10(2)9-13(15(27)25-8-7-24)26-14(16(19,20)17(21,22)23)11-3-5-12(18)6-4-11/h3-6,10,13-14,26H,8-9H2,1-2H3,(H,25,27)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173400
((S)-2-{[(4-Bromo-phenyl)-(4-methanesulfonyl-phenyl...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)c1ccc(cc1)S(C)(=O)=O)C(=O)NCC#N Show InChI InChI=1S/C22H26BrN3O3S/c1-15(2)14-20(22(27)25-13-12-24)26-21(16-4-8-18(23)9-5-16)17-6-10-19(11-7-17)30(3,28)29/h4-11,15,20-21,26H,13-14H2,1-3H3,(H,25,27)/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173403
(4-Methyl-2-[2,2,2-trifluoro-1-(4'-piperazin-1-yl-b...)Show SMILES CC(C)CC(NC(c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173401

(1-[2,2,2-Trifluoro-1-(4'-piperazin-1-yl-biphenyl-4...)Show SMILES FC(F)(F)C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H32F3N5O/c28-27(29,30)24(34-26(12-2-1-3-13-26)25(36)33-15-14-31)22-6-4-20(5-7-22)21-8-10-23(11-9-21)35-18-16-32-17-19-35/h4-11,24,32,34H,1-3,12-13,15-19H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19856
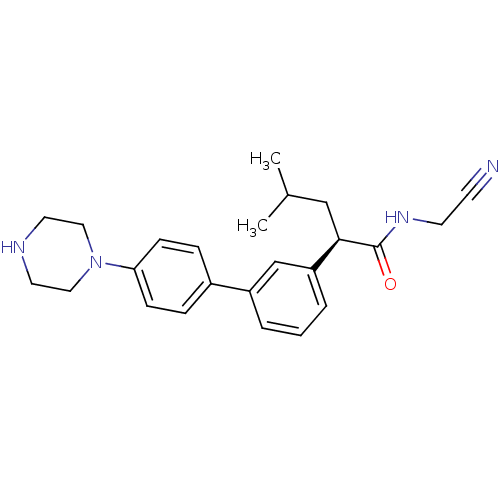
((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...)Show SMILES CC(C)C[C@@H](C(=O)NCC#N)c1cccc(c1)-c1ccc(cc1)N1CCNCC1 |r| Show InChI InChI=1S/C24H30N4O/c1-18(2)16-23(24(29)27-11-10-25)21-5-3-4-20(17-21)19-6-8-22(9-7-19)28-14-12-26-13-15-28/h3-9,17-18,23,26H,11-16H2,1-2H3,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173408
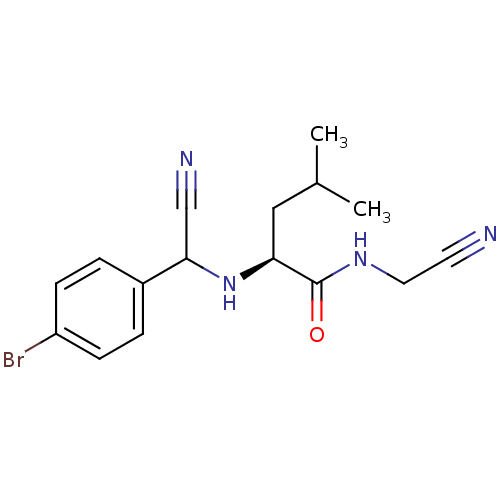
((S)-2-{[(4-Bromo-phenyl)-cyano-methyl]-amino}-4-me...)Show InChI InChI=1S/C16H19BrN4O/c1-11(2)9-14(16(22)20-8-7-18)21-15(10-19)12-3-5-13(17)6-4-12/h3-6,11,14-15,21H,8-9H2,1-2H3,(H,20,22)/t14-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50173405

((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50173403

(4-Methyl-2-[2,2,2-trifluoro-1-(4'-piperazin-1-yl-b...)Show SMILES CC(C)CC(NC(c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50173405

((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 451 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50240981

(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 614 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173402
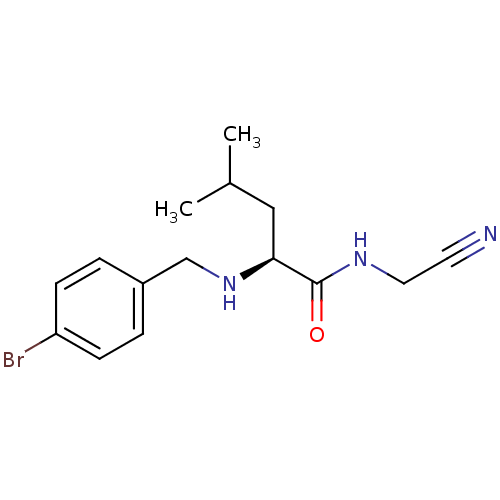
((S)-2-(4-Bromo-benzylamino)-4-methyl-pentanoic aci...)Show InChI InChI=1S/C15H20BrN3O/c1-11(2)9-14(15(20)18-8-7-17)19-10-12-3-5-13(16)6-4-12/h3-6,11,14,19H,8-10H2,1-2H3,(H,18,20)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 802 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50173403

(4-Methyl-2-[2,2,2-trifluoro-1-(4'-piperazin-1-yl-b...)Show SMILES CC(C)CC(NC(c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 902 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50173406

((S)-2-[1-(4-Bromo-phenyl)-ethylamino]-4-methyl-pen...)Show InChI InChI=1S/C16H22BrN3O/c1-11(2)10-15(16(21)19-9-8-18)20-12(3)13-4-6-14(17)7-5-13/h4-7,11-12,15,20H,9-10H2,1-3H3,(H,19,21)/t12?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 988 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K using humanized rabbit enzyme |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19856

((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...)Show SMILES CC(C)C[C@@H](C(=O)NCC#N)c1cccc(c1)-c1ccc(cc1)N1CCNCC1 |r| Show InChI InChI=1S/C24H30N4O/c1-18(2)16-23(24(29)27-11-10-25)21-5-3-4-20(17-21)19-6-8-22(9-7-19)28-14-12-26-13-15-28/h3-9,17-18,23,26H,11-16H2,1-2H3,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50240981

(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50173405

((S)-2-[1-(4-Bromo-phenyl)-2,2,2-trifluoro-ethylami...)Show SMILES CC(C)C[C@H](NC(c1ccc(Br)cc1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C16H19BrF3N3O/c1-10(2)9-13(15(24)22-8-7-21)23-14(16(18,19)20)11-3-5-12(17)6-4-11/h3-6,10,13-14,23H,8-9H2,1-2H3,(H,22,24)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19856

((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...)Show SMILES CC(C)C[C@@H](C(=O)NCC#N)c1cccc(c1)-c1ccc(cc1)N1CCNCC1 |r| Show InChI InChI=1S/C24H30N4O/c1-18(2)16-23(24(29)27-11-10-25)21-5-3-4-20(17-21)19-6-8-22(9-7-19)28-14-12-26-13-15-28/h3-9,17-18,23,26H,11-16H2,1-2H3,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM19856

((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...)Show SMILES CC(C)C[C@@H](C(=O)NCC#N)c1cccc(c1)-c1ccc(cc1)N1CCNCC1 |r| Show InChI InChI=1S/C24H30N4O/c1-18(2)16-23(24(29)27-11-10-25)21-5-3-4-20(17-21)19-6-8-22(9-7-19)28-14-12-26-13-15-28/h3-9,17-18,23,26H,11-16H2,1-2H3,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50173401

(1-[2,2,2-Trifluoro-1-(4'-piperazin-1-yl-biphenyl-4...)Show SMILES FC(F)(F)C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H32F3N5O/c28-27(29,30)24(34-26(12-2-1-3-13-26)25(36)33-15-14-31)22-6-4-20(5-7-22)21-8-10-23(11-9-21)35-18-16-32-17-19-35/h4-11,24,32,34H,1-3,12-13,15-19H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50173403

(4-Methyl-2-[2,2,2-trifluoro-1-(4'-piperazin-1-yl-b...)Show SMILES CC(C)CC(NC(c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50173401

(1-[2,2,2-Trifluoro-1-(4'-piperazin-1-yl-biphenyl-4...)Show SMILES FC(F)(F)C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H32F3N5O/c28-27(29,30)24(34-26(12-2-1-3-13-26)25(36)33-15-14-31)22-6-4-20(5-7-22)21-8-10-23(11-9-21)35-18-16-32-17-19-35/h4-11,24,32,34H,1-3,12-13,15-19H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin B from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50240981

(4'-piperazin-1-yl-biphenyl-4-carboxylic acid [1-(c...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C26H31N5O2/c27-14-15-29-25(33)26(12-2-1-3-13-26)30-24(32)22-6-4-20(5-7-22)21-8-10-23(11-9-21)31-18-16-28-17-19-31/h4-11,28H,1-3,12-13,15-19H2,(H,29,33)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin S from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50173401

(1-[2,2,2-Trifluoro-1-(4'-piperazin-1-yl-biphenyl-4...)Show SMILES FC(F)(F)C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C27H32F3N5O/c28-27(29,30)24(34-26(12-2-1-3-13-26)25(36)33-15-14-31)22-6-4-20(5-7-22)21-8-10-23(11-9-21)35-18-16-32-17-19-35/h4-11,24,32,34H,1-3,12-13,15-19H2,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin L from human |
Bioorg Med Chem Lett 15: 4741-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.071
BindingDB Entry DOI: 10.7270/Q20001NJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data