 Found 44 hits of Enzyme Inhibition Constant Data
Found 44 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206997
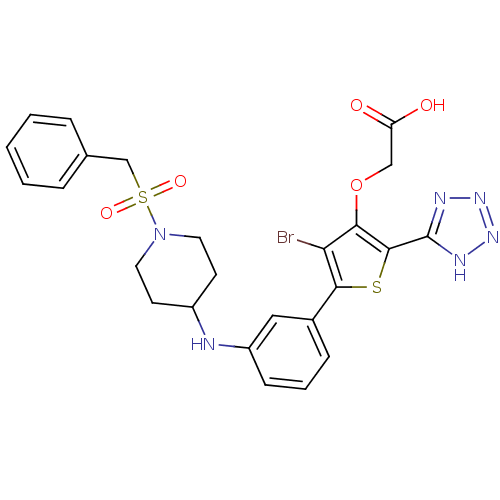
(2-(5-(3-(1-(benzylsulfonyl)piperidin-4-ylamino)phe...)Show SMILES OC(=O)COc1c(Br)c(sc1-c1nnn[nH]1)-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C25H25BrN6O5S2/c26-21-22(37-14-20(33)34)24(25-28-30-31-29-25)38-23(21)17-7-4-8-19(13-17)27-18-9-11-32(12-10-18)39(35,36)15-16-5-2-1-3-6-16/h1-8,13,18,27H,9-12,14-15H2,(H,33,34)(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206991

(2-(5-(3-(1-(benzylsulfonyl)piperidin-4-yloxy)pheny...)Show SMILES Cc1c(OCC(O)=O)c(sc1-c1cccc(OC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1)-c1nnn[nH]1 Show InChI InChI=1S/C26H27N5O6S2/c1-17-23(36-15-22(32)33)25(26-27-29-30-28-26)38-24(17)19-8-5-9-21(14-19)37-20-10-12-31(13-11-20)39(34,35)16-18-6-3-2-4-7-18/h2-9,14,20H,10-13,15-16H2,1H3,(H,32,33)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207008

(2-(4-bromo-5-(3-(3,3,5,5-tetramethylcyclohexylamin...)Show SMILES CC1(C)CC(CC(C)(C)C1)Nc1cccc(c1)-c1sc(c(OCC(O)=O)c1Br)-c1nnn[nH]1 Show InChI InChI=1S/C23H28BrN5O3S/c1-22(2)9-15(10-23(3,4)12-22)25-14-7-5-6-13(8-14)19-17(24)18(32-11-16(30)31)20(33-19)21-26-28-29-27-21/h5-8,15,25H,9-12H2,1-4H3,(H,30,31)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207009

(CHEMBL393538 | N-{3-[3-chloro-4-(1,1,4-trioxo-1,2,...)Show SMILES NS(=O)(=O)N(C1CCCCC1)c1cccc(c1)-c1scc(N2C=C(O)NS2(=O)=O)c1Cl |t:24| Show InChI InChI=1S/C18H21ClN4O5S3/c19-17-15(22-10-16(24)21-31(22,27)28)11-29-18(17)12-5-4-8-14(9-12)23(30(20,25)26)13-6-2-1-3-7-13/h4-5,8-11,13,21,24H,1-3,6-7H2,(H2,20,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206987

(5-{4-methyl-5-[3-(1-phenylmethanesulfonyl-piperidi...)Show SMILES Cc1c(csc1-c1cccc(NC2CCN(CC2)S(=O)(=O)Cc2ccccc2)c1)N1C=C(O)NS1(=O)=O |t:34| Show InChI InChI=1S/C25H28N4O5S3/c1-18-23(29-15-24(30)27-37(29,33)34)16-35-25(18)20-8-5-9-22(14-20)26-21-10-12-28(13-11-21)36(31,32)17-19-6-3-2-4-7-19/h2-9,14-16,21,26-27,30H,10-13,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207003

(2-(4-bromo-5-(3-(N-(3,3,5,5-tetramethylcyclohexyl)...)Show SMILES CC(=O)N(C1CC(C)(C)CC(C)(C)C1)c1cccc(c1)-c1sc(-c2nnn[nH]2)c(OCC(O)=O)c1Br Show InChI InChI=1S/C25H30BrN5O4S/c1-14(32)31(17-10-24(2,3)13-25(4,5)11-17)16-8-6-7-15(9-16)21-19(26)20(35-12-18(33)34)22(36-21)23-27-29-30-28-23/h6-9,17H,10-13H2,1-5H3,(H,33,34)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207024

(5-{5-[3-(cyclohexylamino)phenyl]-4-methylthien-3-y...)Show SMILES Cc1c(csc1-c1cccc(NC2CCCCC2)c1)N1C=C(O)NS1(=O)=O |t:23| Show InChI InChI=1S/C19H23N3O3S2/c1-13-17(22-11-18(23)21-27(22,24)25)12-26-19(13)14-6-5-9-16(10-14)20-15-7-3-2-4-8-15/h5-6,9-12,15,20-21,23H,2-4,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206988

(5-{4-chloro-5-[3-(cyclohexylamino)phenyl]thien-3-y...)Show SMILES OC1=CN(c2csc(c2Cl)-c2cccc(NC3CCCCC3)c2)S(=O)(=O)N1 |t:1| Show InChI InChI=1S/C18H20ClN3O3S2/c19-17-15(22-10-16(23)21-27(22,24)25)11-26-18(17)12-5-4-8-14(9-12)20-13-6-2-1-3-7-13/h4-5,8-11,13,20-21,23H,1-3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206990

(5-{4-methyl-5-[3-(3,3,5,5-tetramethyl-cyclohexylam...)Show SMILES Cc1c(csc1-c1cccc(NC2CC(C)(C)CC(C)(C)C2)c1)N1C=C(O)NS1(=O)=O |t:27| Show InChI InChI=1S/C23H31N3O3S2/c1-15-19(26-12-20(27)25-31(26,28)29)13-30-21(15)16-7-6-8-17(9-16)24-18-10-22(2,3)14-23(4,5)11-18/h6-9,12-13,18,24-25,27H,10-11,14H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207022

(5-(4-CHLORO-5-PHENYL-3-THIENYL)-1,2,5-THIADIAZOLID...)Show SMILES OC1=CN(c2csc(c2Cl)-c2ccccc2)S(=O)(=O)N1 |t:1| Show InChI InChI=1S/C12H9ClN2O3S2/c13-11-9(15-6-10(16)14-20(15,17)18)7-19-12(11)8-4-2-1-3-5-8/h1-7,14,16H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206995
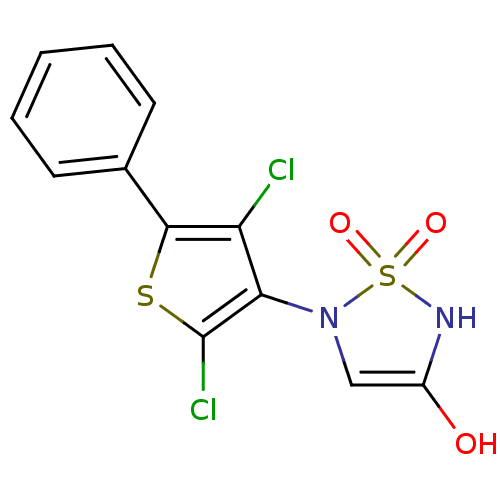
(5-(2,4-dichloro-5-phenylthien-3-yl)-1,2,5-thiadiaz...)Show SMILES OC1=CN(c2c(Cl)sc(c2Cl)-c2ccccc2)S(=O)(=O)N1 |t:1| Show InChI InChI=1S/C12H8Cl2N2O3S2/c13-9-10(16-6-8(17)15-21(16,18)19)12(14)20-11(9)7-4-2-1-3-5-7/h1-6,15,17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207016

(2-(4-bromo-5-(3-(cyclohexylamino)phenyl)-2-(2H-tet...)Show SMILES OC(=O)COc1c(Br)c(sc1-c1nnn[nH]1)-c1cccc(NC2CCCCC2)c1 Show InChI InChI=1S/C19H20BrN5O3S/c20-15-16(28-10-14(26)27)18(19-22-24-25-23-19)29-17(15)11-5-4-8-13(9-11)21-12-6-2-1-3-7-12/h4-5,8-9,12,21H,1-3,6-7,10H2,(H,26,27)(H,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14244
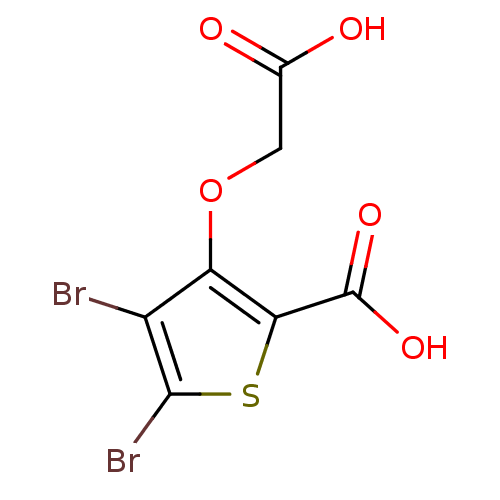
(4,5-dibromo-3-(carboxymethoxy)thiophene-2-carboxyl...)Show InChI InChI=1S/C7H4Br2O5S/c8-3-4(14-1-2(10)11)5(7(12)13)15-6(3)9/h1H2,(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207000
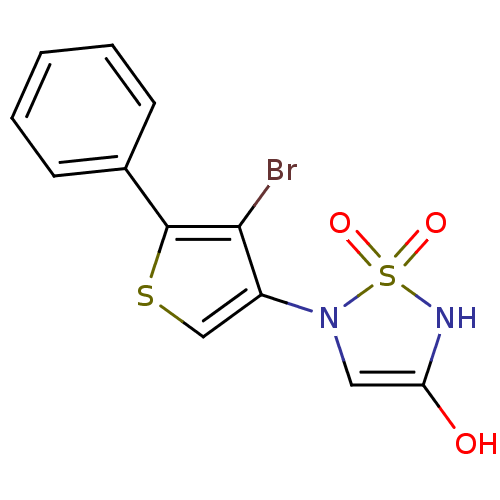
(5-(4-bromo-5-phenylthien-3-yl)-1,2,5-thiadiazolidi...)Show SMILES OC1=CN(c2csc(c2Br)-c2ccccc2)S(=O)(=O)N1 |t:1| Show InChI InChI=1S/C12H9BrN2O3S2/c13-11-9(15-6-10(16)14-20(15,17)18)7-19-12(11)8-4-2-1-3-5-8/h1-7,14,16H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207013

(2-(4-bromo-5-phenyl-2-(2H-tetrazol-5-yl)thiophen-3...)Show InChI InChI=1S/C13H9BrN4O3S/c14-9-10(21-6-8(19)20)12(13-15-17-18-16-13)22-11(9)7-4-2-1-3-5-7/h1-5H,6H2,(H,19,20)(H,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207007

(5-(4-methyl-5-phenylthien-3-yl)-1,2,5-thiadiazolid...)Show SMILES Cc1c(csc1-c1ccccc1)N1C=C(O)NS1(=O)=O |t:15| Show InChI InChI=1S/C13H12N2O3S2/c1-9-11(15-7-12(16)14-20(15,17)18)8-19-13(9)10-5-3-2-4-6-10/h2-8,14,16H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207005
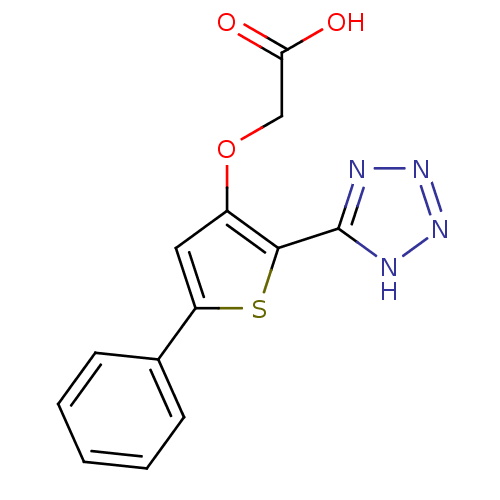
(2-(5-phenyl-2-(2H-tetrazol-5-yl)thiophen-3-yloxy)a...)Show InChI InChI=1S/C13H10N4O3S/c18-11(19)7-20-9-6-10(8-4-2-1-3-5-8)21-12(9)13-14-16-17-15-13/h1-6H,7H2,(H,18,19)(H,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206992
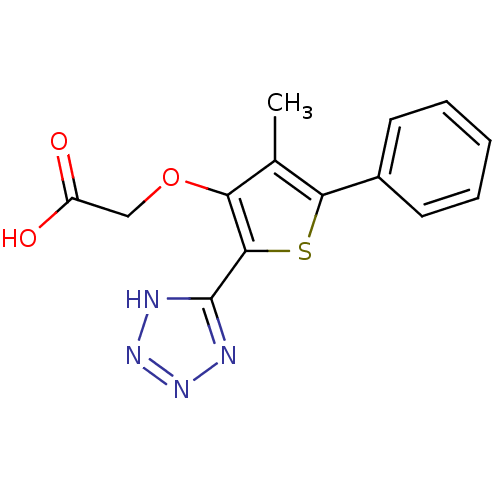
(2-(4-methyl-5-phenyl-2-(2H-tetrazol-5-yl)thiophen-...)Show InChI InChI=1S/C14H12N4O3S/c1-8-11(21-7-10(19)20)13(14-15-17-18-16-14)22-12(8)9-5-3-2-4-6-9/h2-6H,7H2,1H3,(H,19,20)(H,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207014
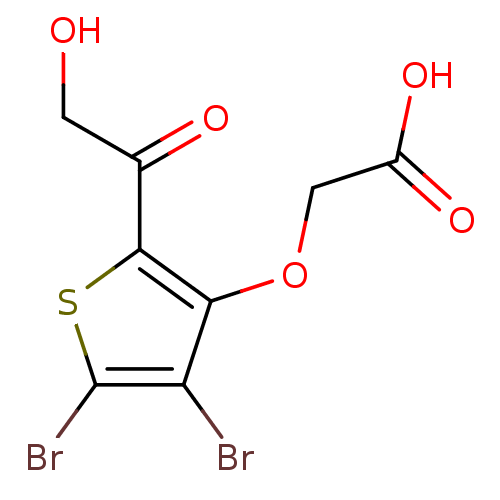
(2-(4,5-dibromo-2-(2-hydroxyacetyl)thiophen-3-yloxy...)Show InChI InChI=1S/C8H6Br2O5S/c9-5-6(15-2-4(13)14)7(3(12)1-11)16-8(5)10/h11H,1-2H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206994

(2-(4,5-dibromo-2-(2H-tetrazol-5-yl)thiophen-3-ylox...)Show InChI InChI=1S/C7H4Br2N4O3S/c8-3-4(16-1-2(14)15)5(17-6(3)9)7-10-12-13-11-7/h1H2,(H,14,15)(H,10,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207000

(5-(4-bromo-5-phenylthien-3-yl)-1,2,5-thiadiazolidi...)Show SMILES OC1=CN(c2csc(c2Br)-c2ccccc2)S(=O)(=O)N1 |t:1| Show InChI InChI=1S/C12H9BrN2O3S2/c13-11-9(15-6-10(16)14-20(15,17)18)7-19-12(11)8-4-2-1-3-5-8/h1-7,14,16H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM14244

(4,5-dibromo-3-(carboxymethoxy)thiophene-2-carboxyl...)Show InChI InChI=1S/C7H4Br2O5S/c8-3-4(14-1-2(10)11)5(7(12)13)15-6(3)9/h1H2,(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207002
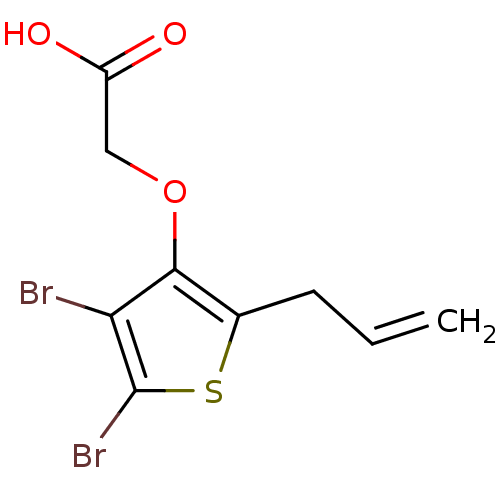
(2-(2-allyl-4,5-dibromothiophen-3-yloxy)acetic acid...)Show InChI InChI=1S/C9H8Br2O3S/c1-2-3-5-8(14-4-6(12)13)7(10)9(11)15-5/h2H,1,3-4H2,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207011

(2-(4,5-dibromo-2-(morpholine-4-carbonyl)thiophen-3...)Show InChI InChI=1S/C11H11Br2NO5S/c12-7-8(19-5-6(15)16)9(20-10(7)13)11(17)14-1-3-18-4-2-14/h1-5H2,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207014

(2-(4,5-dibromo-2-(2-hydroxyacetyl)thiophen-3-yloxy...)Show InChI InChI=1S/C8H6Br2O5S/c9-5-6(15-2-4(13)14)7(3(12)1-11)16-8(5)10/h11H,1-2H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206994

(2-(4,5-dibromo-2-(2H-tetrazol-5-yl)thiophen-3-ylox...)Show InChI InChI=1S/C7H4Br2N4O3S/c8-3-4(16-1-2(14)15)5(17-6(3)9)7-10-12-13-11-7/h1H2,(H,14,15)(H,10,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207015
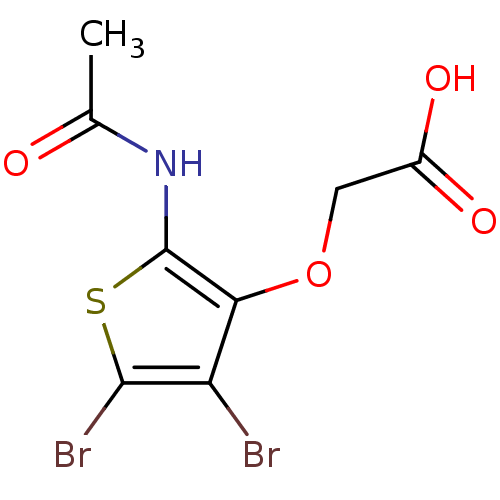
(2-(2-acetamido-4,5-dibromothiophen-3-yloxy)acetic ...)Show InChI InChI=1S/C8H7Br2NO4S/c1-3(12)11-8-6(15-2-4(13)14)5(9)7(10)16-8/h2H2,1H3,(H,11,12)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207023

(2-(4,5-dibromo-2-carbamoylthiophen-3-yloxy)acetic ...)Show InChI InChI=1S/C7H5Br2NO4S/c8-3-4(14-1-2(11)12)5(7(10)13)15-6(3)9/h1H2,(H2,10,13)(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207010
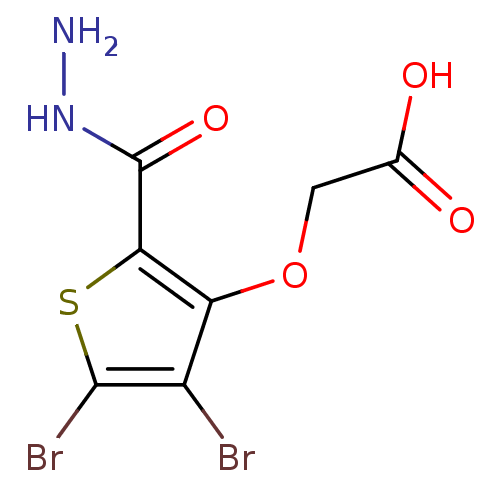
(CHEMBL239577 | {[4,5-dibromo-2-(hydrazinocarbonyl)...)Show InChI InChI=1S/C7H6Br2N2O4S/c8-3-4(15-1-2(12)13)5(7(14)11-10)16-6(3)9/h1,10H2,(H,11,14)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207021

(4,5-dibromo-3-(2,3-dihydroxypropoxy)thiophene-2-ca...)Show InChI InChI=1S/C8H8Br2O5S/c9-4-5(15-2-3(12)1-11)6(8(13)14)16-7(4)10/h3,11-12H,1-2H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206989

(2-(2-((1H-pyrazol-5-yl)carbamoyl)-4,5-dibromothiop...)Show InChI InChI=1S/C10H7Br2N3O4S/c11-6-7(19-3-5(16)17)8(20-9(6)12)10(18)14-4-1-2-13-15-4/h1-2H,3H2,(H,16,17)(H2,13,14,15,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207017
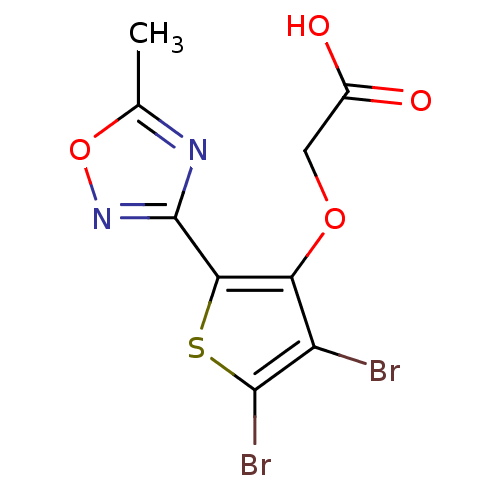
(2-(4,5-dibromo-2-(5-methyl-1,2,4-oxadiazol-3-yl)th...)Show InChI InChI=1S/C9H6Br2N2O4S/c1-3-12-9(13-17-3)7-6(16-2-4(14)15)5(10)8(11)18-7/h2H2,1H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207019
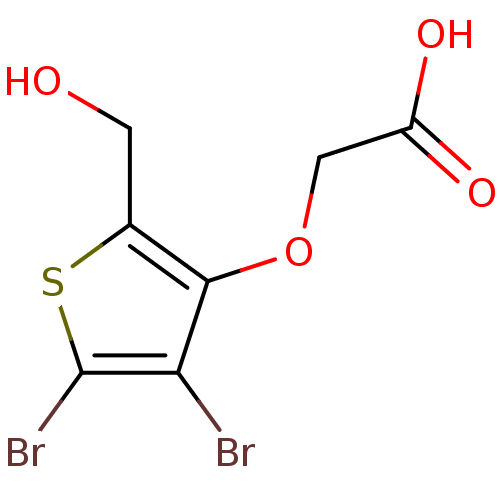
(2-(4,5-dibromo-2-(hydroxymethyl)thiophen-3-yloxy)a...)Show InChI InChI=1S/C7H6Br2O4S/c8-5-6(13-2-4(11)12)3(1-10)14-7(5)9/h10H,1-2H2,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207004

(3-((2H-tetrazol-5-yl)methoxy)-4,5-dibromothiophene...)Show InChI InChI=1S/C7H4Br2N4O3S/c8-3-4(5(7(14)15)17-6(3)9)16-1-2-10-12-13-11-2/h1H2,(H,14,15)(H,10,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206998
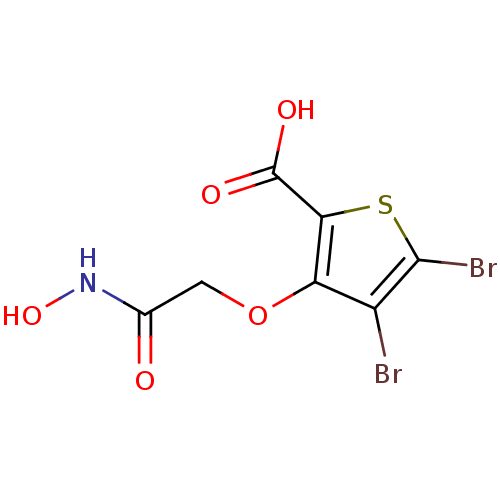
(4,5-dibromo-3-(2-(hydroxyamino)-2-oxoethoxy)thioph...)Show InChI InChI=1S/C7H5Br2NO5S/c8-3-4(15-1-2(11)10-14)5(7(12)13)16-6(3)9/h14H,1H2,(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206993

(2-(4,5-dibromothiophen-3-yloxy)acetic acid | CHEMB...)Show InChI InChI=1S/C6H4Br2O3S/c7-5-3(2-12-6(5)8)11-1-4(9)10/h2H,1H2,(H,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207020
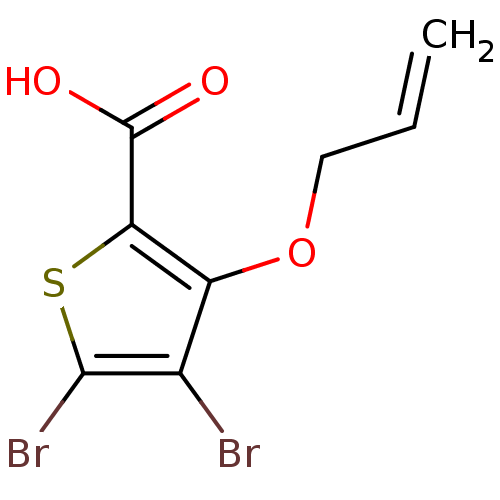
(3-(allyloxy)-4,5-dibromothiophene-2-carboxylic aci...)Show InChI InChI=1S/C8H6Br2O3S/c1-2-3-13-5-4(9)7(10)14-6(5)8(11)12/h2H,1,3H2,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206996

(2-(4,5-dibromo-2-(thiazol-2-ylcarbamoyl)thiophen-3...)Show InChI InChI=1S/C10H6Br2N2O4S2/c11-5-6(18-3-4(15)16)7(20-8(5)12)9(17)14-10-13-1-2-19-10/h1-2H,3H2,(H,15,16)(H,13,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206999
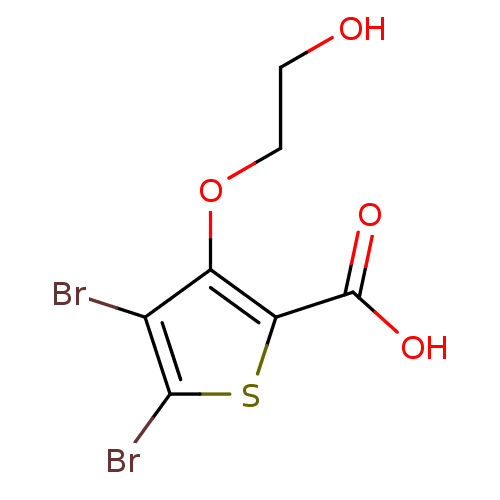
(4,5-dibromo-3-(2-hydroxyethoxy)thiophene-2-carboxy...)Show InChI InChI=1S/C7H6Br2O4S/c8-3-4(13-2-1-10)5(7(11)12)14-6(3)9/h10H,1-2H2,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50206986

(2-(4,5-dibromo-2-(5-isopropyl-1,2,4-oxadiazol-3-yl...)Show InChI InChI=1S/C11H10Br2N2O4S/c1-4(2)11-14-10(15-19-11)8-7(18-3-5(16)17)6(12)9(13)20-8/h4H,3H2,1-2H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207012

(4,5-dibromo-3-(2,2,2-trifluoroethoxy)thiophene-2-c...)Show InChI InChI=1S/C7H3Br2F3O3S/c8-2-3(15-1-7(10,11)12)4(6(13)14)16-5(2)9/h1H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207001

(2-(4,5-dibromo-2-(5-ethyl-1,2,4-oxadiazol-3-yl)thi...)Show InChI InChI=1S/C10H8Br2N2O4S/c1-2-4-13-10(14-18-4)8-7(17-3-5(15)16)6(11)9(12)19-8/h2-3H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207018

(4,5-dibromo-3-(cyanomethoxy)thiophene-2-carboxylic...)Show InChI InChI=1S/C7H3Br2NO3S/c8-3-4(13-2-1-10)5(7(11)12)14-6(3)9/h2H2,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50207006

(2-(4,5-dibromo-2-cyanothiophen-3-yloxy)acetic acid...)Show InChI InChI=1S/C7H3Br2NO3S/c8-5-6(13-2-4(11)12)3(1-10)14-7(5)9/h2H2,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as pNPP hydrolysis |
Bioorg Med Chem Lett 17: 2913-20 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.043
BindingDB Entry DOI: 10.7270/Q29S1QPC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data