

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM26104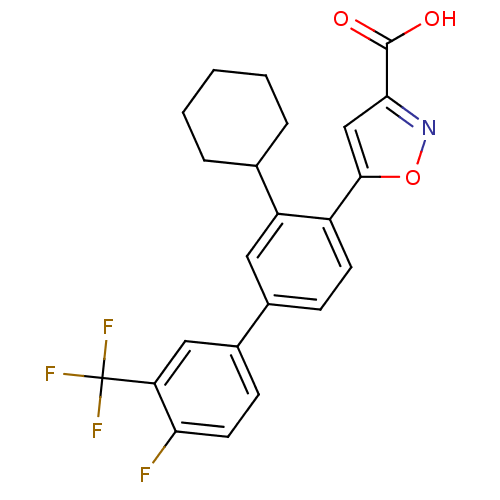 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | -37.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM26103 (5-{4-[4-fluoro-3-(trifluoromethyl)phenyl]-2-(propa...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM26102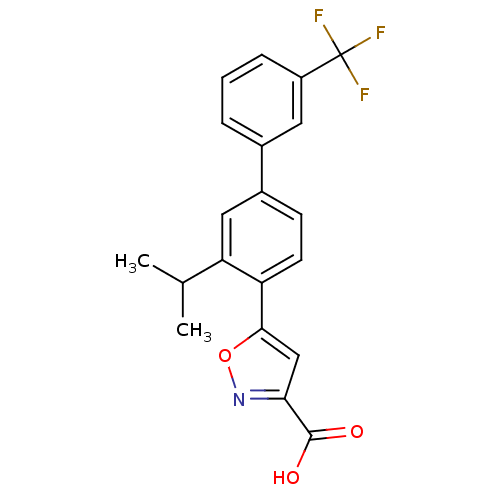 (5-[2-(propan-2-yl)-4-[3-(trifluoromethyl)phenyl]ph...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 850 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM26101 (5-{4-[3-(trifluoromethyl)phenyl]phenyl}-1,2-oxazol...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM26104 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.70E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM26099 (5-{4-[3-(trifluoromethyl)phenyl]phenyl}-1,2-thiazo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM26104 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.14E+4 | -26.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM26104 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM26104 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM26104 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||