

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17786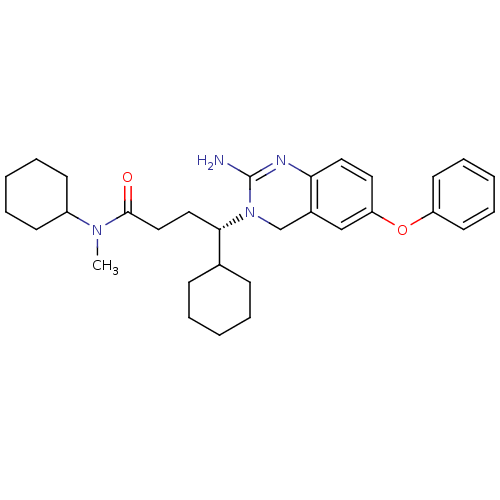 ((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17785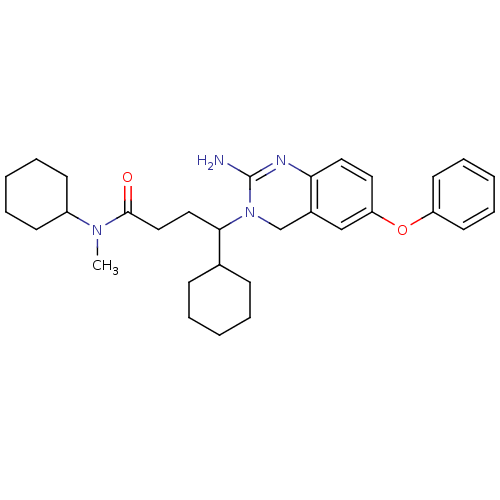 (2-aminoquinazoline, 3 | 4-(2-amino-6-phenoxy-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17784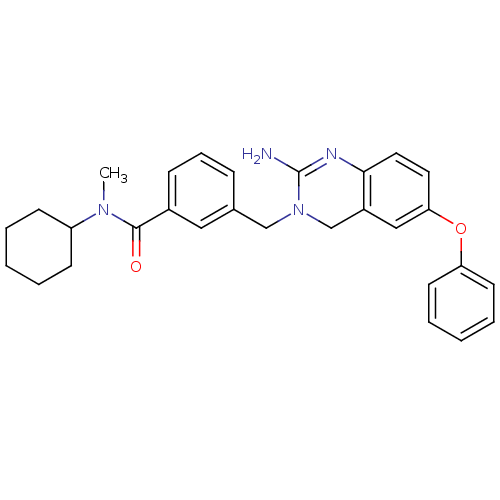 (2-aminoquinazoline, 2 | 3-[(2-amino-6-phenoxy-3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 158 | -38.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM17783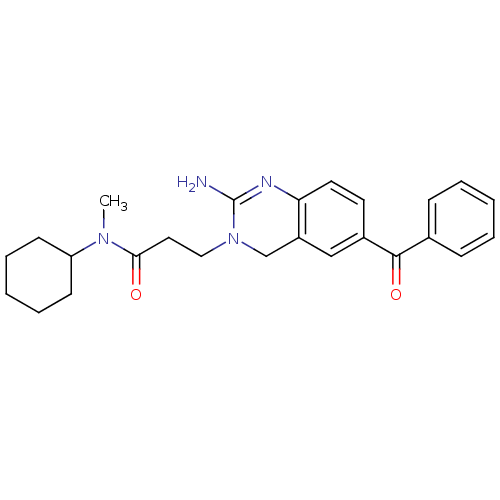 (2-aminoquinazoline, 1 | 3-(2-amino-6-benzoyl-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 900 | -34.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM17786 ((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 4.0 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Cathepsin D activity was measured at pH 4 using a FRET peptide substrate. Compounds were preincubated with recombinant human liver cathepsin D for 20... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17786 ((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Renin activity was measured at pH 7.4 using a FRET peptide substrate. Compounds were added to recombinant human rennin and mixed before substrate was... | J Med Chem 50: 4261-4 (2007) Article DOI: 10.1021/jm0705408 BindingDB Entry DOI: 10.7270/Q24M92T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||