 Found 57 hits of Enzyme Inhibition Constant Data
Found 57 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227724

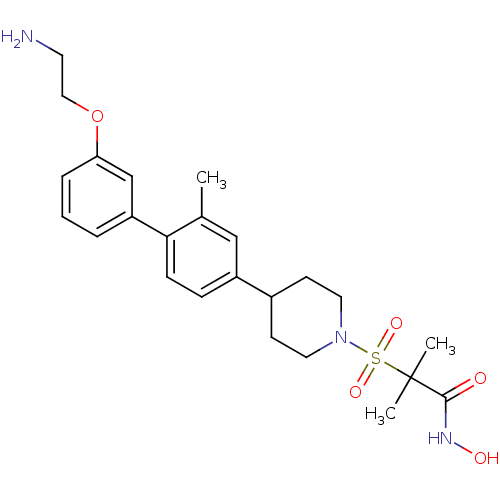
(CHEMBL400083 | N-hydroxy-2-methyl-2-{4-[2-methyl-3...)Show SMILES CNCCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C25H35N3O5S/c1-18-16-20(8-9-23(18)21-6-5-7-22(17-21)33-15-12-26-4)19-10-13-28(14-11-19)34(31,32)25(2,3)24(29)27-30/h5-9,16-17,19,26,30H,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227723
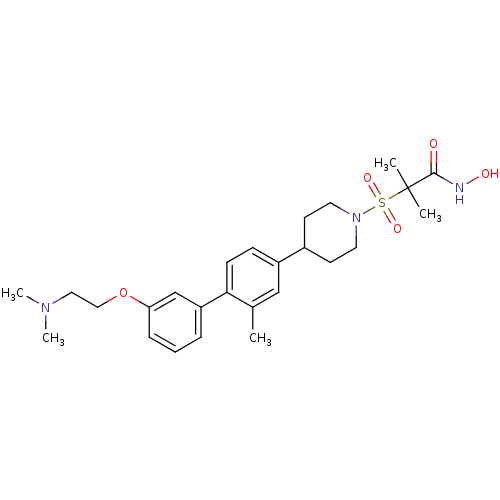
(2-{4-[3'-(2-amino-ethoxy)-2-methyl-biphenyl-4-yl]-...)Show SMILES Cc1cc(ccc1-c1cccc(OCCN)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H33N3O5S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-11-25)18-9-12-27(13-10-18)33(30,31)24(2,3)23(28)26-29/h4-8,15-16,18,29H,9-14,25H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227725
(2-{4-[3'-(2-dimethylamino-ethoxy)-2-methyl-bipheny...)Show SMILES CN(C)CCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C26H37N3O5S/c1-19-17-21(9-10-24(19)22-7-6-8-23(18-22)34-16-15-28(4)5)20-11-13-29(14-12-20)35(32,33)26(2,3)25(30)27-31/h6-10,17-18,20,31H,11-16H2,1-5H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227711

(2-[4-(3'-ethoxy-2-methyl-biphenyl-4-yl)-piperidine...)Show SMILES CCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O5S/c1-5-31-21-8-6-7-20(16-21)22-10-9-19(15-17(22)2)18-11-13-26(14-12-18)32(29,30)24(3,4)23(27)25-28/h6-10,15-16,18,28H,5,11-14H2,1-4H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227717

(CHEMBL251916 | N-hydroxy-2-{4-[3'-(2-methoxy-ethox...)Show SMILES COCCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C25H34N2O6S/c1-18-16-20(8-9-23(18)21-6-5-7-22(17-21)33-15-14-32-4)19-10-12-27(13-11-19)34(30,31)25(2,3)24(28)26-29/h5-9,16-17,19,29H,10-15H2,1-4H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227733
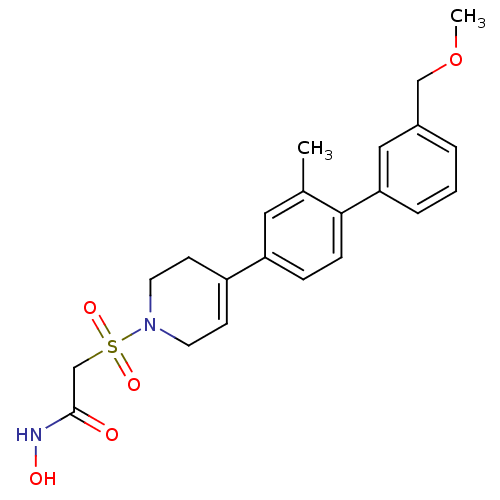
(CHEMBL255030 | N-hydroxy-2-[4-(3'-methoxymethyl-2-...)Show SMILES COCc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:18| Show InChI InChI=1S/C22H26N2O5S/c1-16-12-19(6-7-21(16)20-5-3-4-17(13-20)14-29-2)18-8-10-24(11-9-18)30(27,28)15-22(25)23-26/h3-8,12-13,26H,9-11,14-15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227728

(2-[4-(2-fluoro-biphenyl-4-yl)-3,6-dihydro-2H-pyrid...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(c(F)c1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H19FN2O4S/c20-18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-22(11-9-14)27(25,26)13-19(23)21-24/h1-8,12,24H,9-11,13H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227721
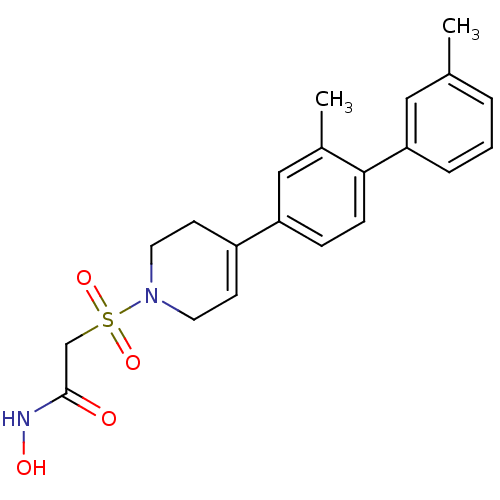
(2-[4-(2,3'-dimethyl-biphenyl-4-yl)-3,6-dihydro-2H-...)Show SMILES Cc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:16| Show InChI InChI=1S/C21H24N2O4S/c1-15-4-3-5-19(12-15)20-7-6-18(13-16(20)2)17-8-10-23(11-9-17)28(26,27)14-21(24)22-25/h3-8,12-13,25H,9-11,14H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227710

(2-[4-(3'-ethoxy-2-methyl-biphenyl-4-yl)-3,6-dihydr...)Show SMILES CCOc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:18| Show InChI InChI=1S/C22H26N2O5S/c1-3-29-20-6-4-5-19(14-20)21-8-7-18(13-16(21)2)17-9-11-24(12-10-17)30(27,28)15-22(25)23-26/h4-9,13-14,26H,3,10-12,15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227734

(CHEMBL254817 | N-hydroxy-2-[4-(3'-methoxy-2-methyl...)Show SMILES COc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:17| Show InChI InChI=1S/C21H24N2O5S/c1-15-12-17(6-7-20(15)18-4-3-5-19(13-18)28-2)16-8-10-23(11-9-16)29(26,27)14-21(24)22-25/h3-8,12-13,25H,9-11,14H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227715

(2-(4-biphenyl-4-yl-3,6-dihydro-2H-pyridine-1-sulfo...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(cc1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H20N2O4S/c22-19(20-23)14-26(24,25)21-12-10-18(11-13-21)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-10,23H,11-14H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227715

(2-(4-biphenyl-4-yl-3,6-dihydro-2H-pyridine-1-sulfo...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(cc1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H20N2O4S/c22-19(20-23)14-26(24,25)21-12-10-18(11-13-21)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-10,23H,11-14H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227719

(CHEMBL254606 | N-hydroxy-2-[4-(2-methyl-biphenyl-4...)Show SMILES Cc1cc(ccc1-c1ccccc1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:15| Show InChI InChI=1S/C20H22N2O4S/c1-15-13-18(7-8-19(15)17-5-3-2-4-6-17)16-9-11-22(12-10-16)27(25,26)14-20(23)21-24/h2-9,13,24H,10-12,14H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227728

(2-[4-(2-fluoro-biphenyl-4-yl)-3,6-dihydro-2H-pyrid...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(c(F)c1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H19FN2O4S/c20-18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-22(11-9-14)27(25,26)13-19(23)21-24/h1-8,12,24H,9-11,13H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227714

(2-(4-biphenyl-4-yl-piperidine-1-sulfonyl)-N-hydrox...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(CC1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C19H22N2O4S/c22-19(20-23)14-26(24,25)21-12-10-18(11-13-21)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-9,18,23H,10-14H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227727
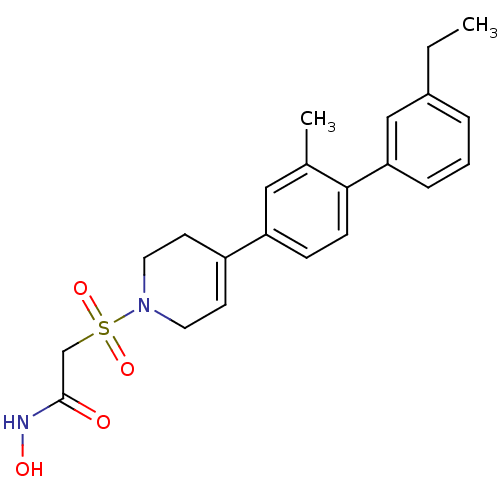
(2-[4-(3'-ethyl-2-methyl-biphenyl-4-yl)-3,6-dihydro...)Show SMILES CCc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:17| Show InChI InChI=1S/C22H26N2O4S/c1-3-17-5-4-6-20(14-17)21-8-7-19(13-16(21)2)18-9-11-24(12-10-18)29(27,28)15-22(25)23-26/h4-9,13-14,26H,3,10-12,15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227730

(CHEMBL398640 | N-hydroxy-2-[4-(2-methyl-3'-trifluo...)Show SMILES Cc1cc(ccc1-c1cccc(OC(F)(F)F)c1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:20| Show InChI InChI=1S/C21H21F3N2O5S/c1-14-11-16(15-7-9-26(10-8-15)32(29,30)13-20(27)25-28)5-6-19(14)17-3-2-4-18(12-17)31-21(22,23)24/h2-7,11-12,28H,8-10,13H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227716
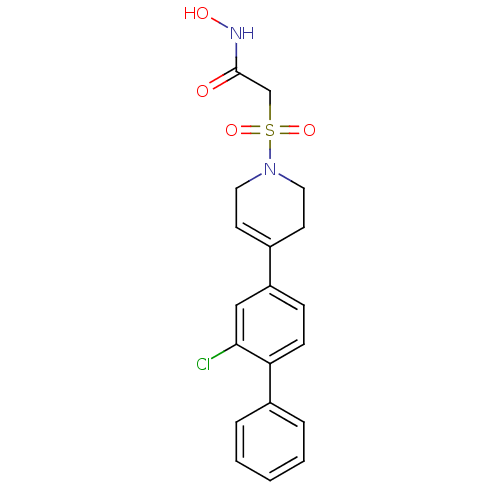
(2-[4-(2-chloro-biphenyl-4-yl)-3,6-dihydro-2H-pyrid...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(c(Cl)c1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H19ClN2O4S/c20-18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-22(11-9-14)27(25,26)13-19(23)21-24/h1-8,12,24H,9-11,13H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227732

(2-[3-(biphenyl-4-yloxy)-azetidine-1-sulfonyl]-N-hy...)Show SMILES ONC(=O)CS(=O)(=O)N1CC(C1)Oc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C17H18N2O5S/c20-17(18-21)12-25(22,23)19-10-16(11-19)24-15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-9,16,21H,10-12H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227731
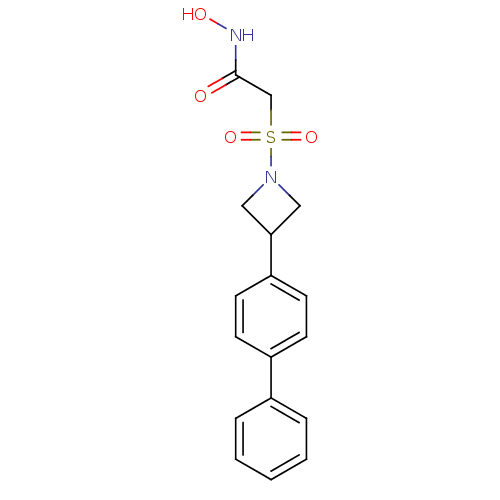
(2-(3-biphenyl-4-yl-azetidine-1-sulfonyl)-N-hydroxy...)Show SMILES ONC(=O)CS(=O)(=O)N1CC(C1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C17H18N2O4S/c20-17(18-21)12-24(22,23)19-10-16(11-19)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-9,16,21H,10-12H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227726
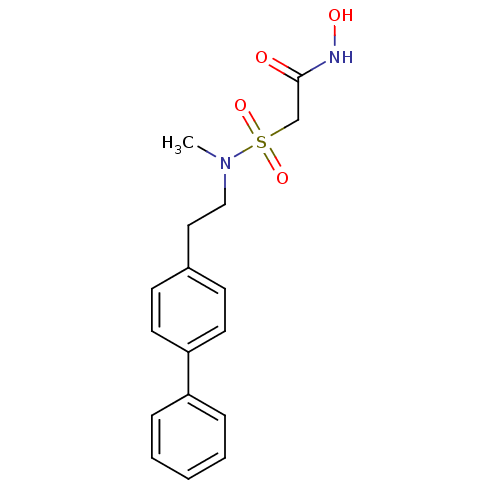
(2-[(2-biphenyl-4-yl-ethyl)-methyl-sulfamoyl]-N-hyd...)Show InChI InChI=1S/C17H20N2O4S/c1-19(24(22,23)13-17(20)18-21)12-11-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,21H,11-13H2,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227730

(CHEMBL398640 | N-hydroxy-2-[4-(2-methyl-3'-trifluo...)Show SMILES Cc1cc(ccc1-c1cccc(OC(F)(F)F)c1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:20| Show InChI InChI=1S/C21H21F3N2O5S/c1-14-11-16(15-7-9-26(10-8-15)32(29,30)13-20(27)25-28)5-6-19(14)17-3-2-4-18(12-17)31-21(22,23)24/h2-7,11-12,28H,8-10,13H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227720
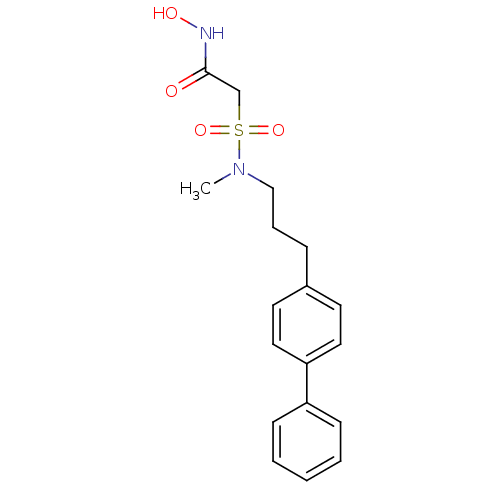
(2-[(3-biphenyl-4-yl-propyl)-methyl-sulfamoyl]-N-hy...)Show SMILES CN(CCCc1ccc(cc1)-c1ccccc1)S(=O)(=O)CC(=O)NO Show InChI InChI=1S/C18H22N2O4S/c1-20(25(23,24)14-18(21)19-22)13-5-6-15-9-11-17(12-10-15)16-7-3-2-4-8-16/h2-4,7-12,22H,5-6,13-14H2,1H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227723

(2-{4-[3'-(2-amino-ethoxy)-2-methyl-biphenyl-4-yl]-...)Show SMILES Cc1cc(ccc1-c1cccc(OCCN)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H33N3O5S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-11-25)18-9-12-27(13-10-18)33(30,31)24(2,3)23(28)26-29/h4-8,15-16,18,29H,9-14,25H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227724

(CHEMBL400083 | N-hydroxy-2-methyl-2-{4-[2-methyl-3...)Show SMILES CNCCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C25H35N3O5S/c1-18-16-20(8-9-23(18)21-6-5-7-22(17-21)33-15-12-26-4)19-10-13-28(14-11-19)34(31,32)25(2,3)24(29)27-30/h5-9,16-17,19,26,30H,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227733

(CHEMBL255030 | N-hydroxy-2-[4-(3'-methoxymethyl-2-...)Show SMILES COCc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:18| Show InChI InChI=1S/C22H26N2O5S/c1-16-12-19(6-7-21(16)20-5-3-4-17(13-20)14-29-2)18-8-10-24(11-9-18)30(27,28)15-22(25)23-26/h3-8,12-13,26H,9-11,14-15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227729
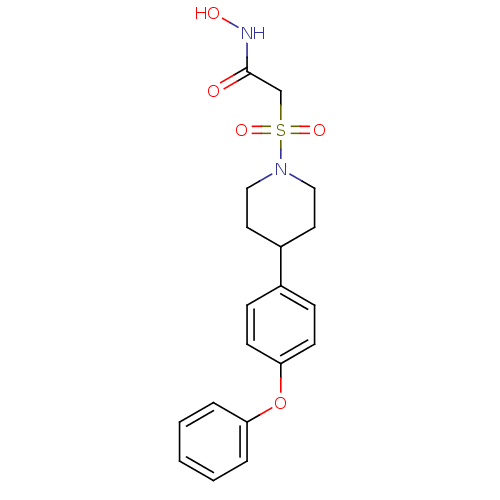
(CHEMBL400463 | N-hydroxy-2-(4-(4-phenoxyphenyl)pip...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(CC1)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O5S/c22-19(20-23)14-27(24,25)21-12-10-16(11-13-21)15-6-8-18(9-7-15)26-17-4-2-1-3-5-17/h1-9,16,23H,10-14H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227734

(CHEMBL254817 | N-hydroxy-2-[4-(3'-methoxy-2-methyl...)Show SMILES COc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:17| Show InChI InChI=1S/C21H24N2O5S/c1-15-12-17(6-7-20(15)18-4-3-5-19(13-18)28-2)16-8-10-23(11-9-16)29(26,27)14-21(24)22-25/h3-8,12-13,25H,9-11,14H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227719

(CHEMBL254606 | N-hydroxy-2-[4-(2-methyl-biphenyl-4...)Show SMILES Cc1cc(ccc1-c1ccccc1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:15| Show InChI InChI=1S/C20H22N2O4S/c1-15-13-18(7-8-19(15)17-5-3-2-4-6-17)16-9-11-22(12-10-16)27(25,26)14-20(23)21-24/h2-9,13,24H,10-12,14H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227735
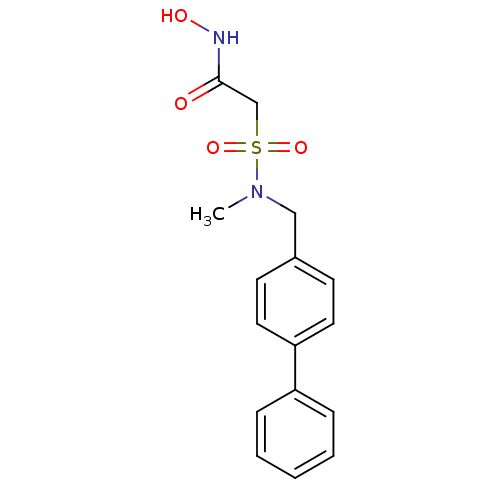
(2-(biphenyl-4-ylmethyl-methyl-sulfamoyl)-N-hydroxy...)Show InChI InChI=1S/C16H18N2O4S/c1-18(23(21,22)12-16(19)17-20)11-13-7-9-15(10-8-13)14-5-3-2-4-6-14/h2-10,20H,11-12H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227713
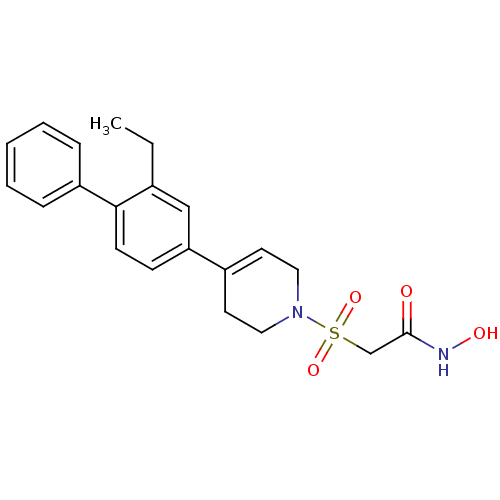
(2-[4-(2-ethyl-biphenyl-4-yl)-3,6-dihydro-2H-pyridi...)Show SMILES CCc1cc(ccc1-c1ccccc1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:16| Show InChI InChI=1S/C21H24N2O4S/c1-2-16-14-19(8-9-20(16)18-6-4-3-5-7-18)17-10-12-23(13-11-17)28(26,27)15-21(24)22-25/h3-10,14,25H,2,11-13,15H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227711

(2-[4-(3'-ethoxy-2-methyl-biphenyl-4-yl)-piperidine...)Show SMILES CCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O5S/c1-5-31-21-8-6-7-20(16-21)22-10-9-19(15-17(22)2)18-11-13-26(14-12-18)32(29,30)24(3,4)23(27)25-28/h6-10,15-16,18,28H,5,11-14H2,1-4H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 529 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227725

(2-{4-[3'-(2-dimethylamino-ethoxy)-2-methyl-bipheny...)Show SMILES CN(C)CCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C26H37N3O5S/c1-19-17-21(9-10-24(19)22-7-6-8-23(18-22)34-16-15-28(4)5)20-11-13-29(14-12-20)35(32,33)26(2,3)25(30)27-31/h6-10,17-18,20,31H,11-16H2,1-5H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227716

(2-[4-(2-chloro-biphenyl-4-yl)-3,6-dihydro-2H-pyrid...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(c(Cl)c1)-c1ccccc1 |c:11| Show InChI InChI=1S/C19H19ClN2O4S/c20-18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-22(11-9-14)27(25,26)13-19(23)21-24/h1-8,12,24H,9-11,13H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227721

(2-[4-(2,3'-dimethyl-biphenyl-4-yl)-3,6-dihydro-2H-...)Show SMILES Cc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:16| Show InChI InChI=1S/C21H24N2O4S/c1-15-4-3-5-19(12-15)20-7-6-18(13-16(20)2)17-8-10-23(11-9-17)28(26,27)14-21(24)22-25/h3-8,12-13,25H,9-11,14H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227717

(CHEMBL251916 | N-hydroxy-2-{4-[3'-(2-methoxy-ethox...)Show SMILES COCCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C25H34N2O6S/c1-18-16-20(8-9-23(18)21-6-5-7-22(17-21)33-15-14-32-4)19-10-12-27(13-11-19)34(30,31)25(2,3)24(28)26-29/h5-9,16-17,19,29H,10-15H2,1-4H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 853 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227712
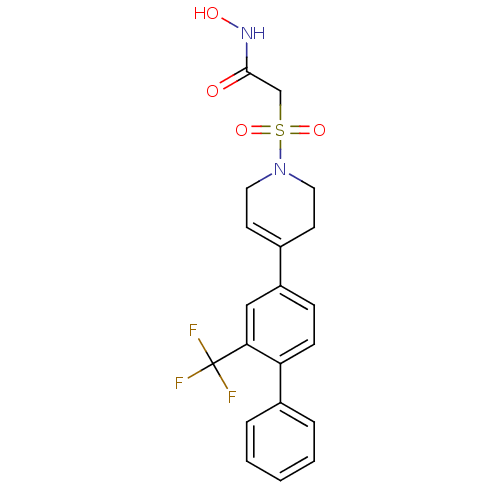
(CHEMBL254816 | N-hydroxy-2-[4-(2-trifluoromethyl-b...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(-c2ccccc2)c(c1)C(F)(F)F |c:11| Show InChI InChI=1S/C20H19F3N2O4S/c21-20(22,23)18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-25(11-9-14)30(28,29)13-19(26)24-27/h1-8,12,27H,9-11,13H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227710

(2-[4-(3'-ethoxy-2-methyl-biphenyl-4-yl)-3,6-dihydr...)Show SMILES CCOc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:18| Show InChI InChI=1S/C22H26N2O5S/c1-3-29-20-6-4-5-19(14-20)21-8-7-18(13-16(21)2)17-9-11-24(12-10-17)30(27,28)15-22(25)23-26/h4-9,13-14,26H,3,10-12,15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 998 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227727

(2-[4-(3'-ethyl-2-methyl-biphenyl-4-yl)-3,6-dihydro...)Show SMILES CCc1cccc(c1)-c1ccc(cc1C)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:17| Show InChI InChI=1S/C22H26N2O4S/c1-3-17-5-4-6-20(14-17)21-8-7-19(13-16(21)2)18-9-11-24(12-10-18)29(27,28)15-22(25)23-26/h4-9,13-14,26H,3,10-12,15H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227712

(CHEMBL254816 | N-hydroxy-2-[4-(2-trifluoromethyl-b...)Show SMILES ONC(=O)CS(=O)(=O)N1CCC(=CC1)c1ccc(-c2ccccc2)c(c1)C(F)(F)F |c:11| Show InChI InChI=1S/C20H19F3N2O4S/c21-20(22,23)18-12-16(6-7-17(18)15-4-2-1-3-5-15)14-8-10-25(11-9-14)30(28,29)13-19(26)24-27/h1-8,12,27H,9-11,13H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227713

(2-[4-(2-ethyl-biphenyl-4-yl)-3,6-dihydro-2H-pyridi...)Show SMILES CCc1cc(ccc1-c1ccccc1)C1=CCN(CC1)S(=O)(=O)CC(=O)NO |t:16| Show InChI InChI=1S/C21H24N2O4S/c1-2-16-14-19(8-9-20(16)18-6-4-3-5-7-18)17-10-12-23(13-11-17)28(26,27)15-21(24)22-25/h3-10,14,25H,2,11-13,15H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50227718

((R)-3-((S)-1-((S)-2-methoxy-1-phenylethylamino)-3,...)Show SMILES COC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C35H44N2O5/c1-24-21-25(19-20-29(24)26-14-8-6-9-15-26)13-12-18-28(22-31(38)39)33(40)37-32(35(2,3)4)34(41)36-30(23-42-5)27-16-10-7-11-17-27/h6-11,14-17,19-21,28,30,32H,12-13,18,22-23H2,1-5H3,(H,36,41)(H,37,40)(H,38,39)/t28-,30-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data