

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237869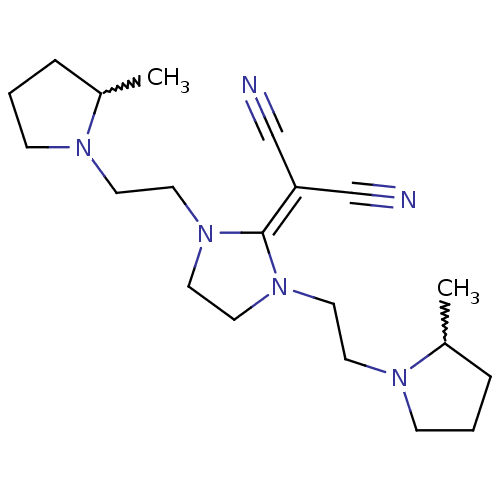 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237874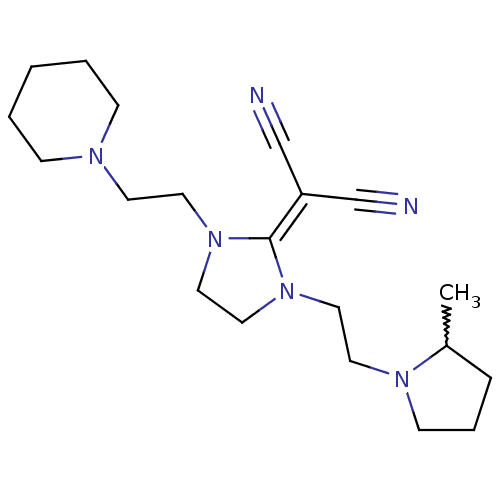 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237872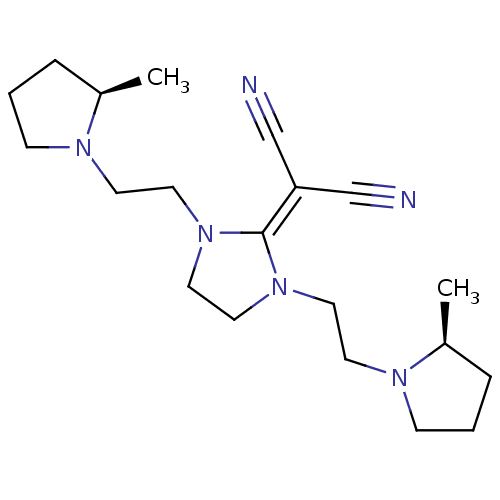 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237869 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237874 ((+/-)-2-(1-(2-(2-methylpyrrolidin-1-yl)ethyl)-3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237872 (2-(3-(2-((R)-2-methylpyrrolidin-1-yl)ethyl)-1-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237875 (2-(1,3-bis(2-((R)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237878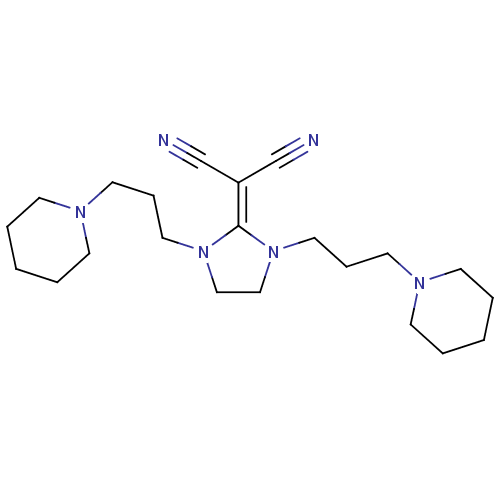 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237878 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237871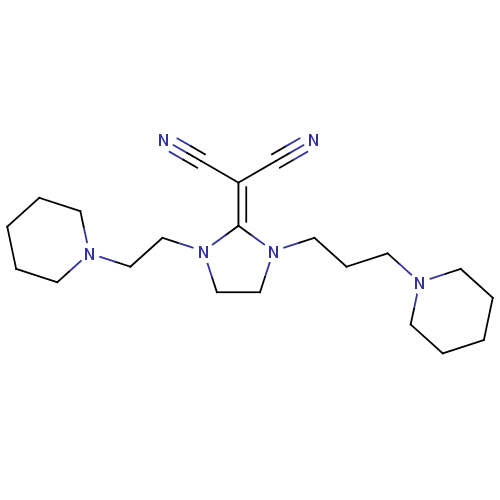 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237871 (2-(1-(2-(piperidin-1-yl)ethyl)-3-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237873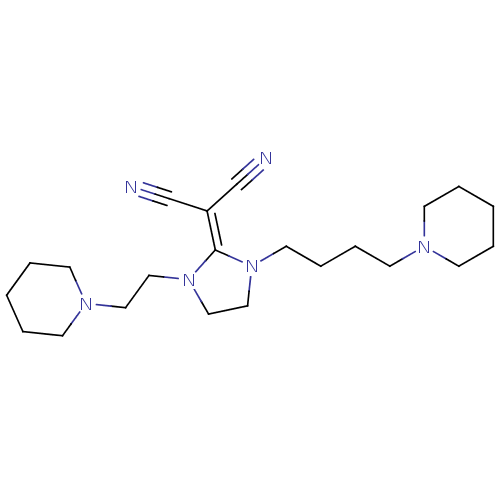 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237873 (2-(1-(4-(piperidin-1-yl)butyl)-3-(2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237879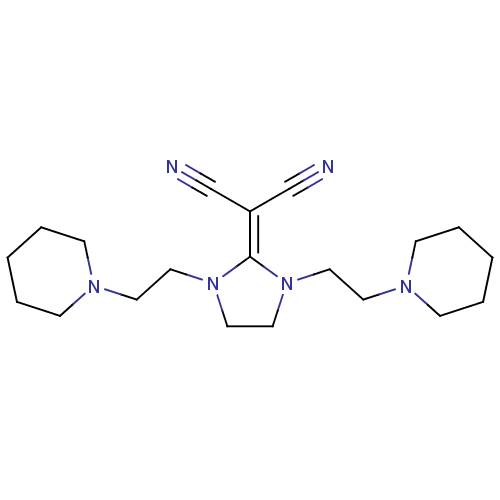 (2-(1,3-bis(2-(piperidin-1-yl)ethyl)imidazolidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237876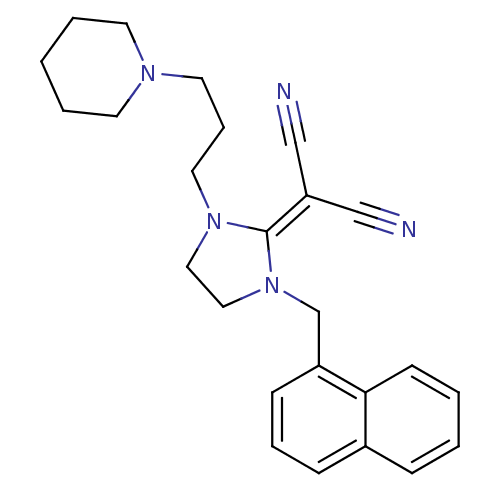 (2-(1-(naphthalen-1-ylmethyl)-3-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237879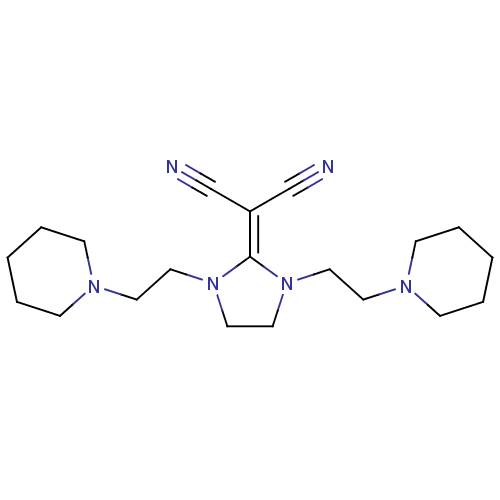 (2-(1,3-bis(2-(piperidin-1-yl)ethyl)imidazolidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237877 (2-(1-(2-((3-(dimethylamino)propyl)(ethyl)amino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50237877 (2-(1-(2-((3-(dimethylamino)propyl)(ethyl)amino)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in monkey COS7 cells | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237876 (2-(1-(naphthalen-1-ylmethyl)-3-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from histamine H3 receptor in rat striatal membrane | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237870 (2-(1,3-bis(2-((S)-2-methylpyrrolidin-1-yl)ethyl)im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat forebrain assessed as inhibition of [3H]histamine release from synaptosomes | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237869 ((+/-)-2-(1,3-bis(2-(2-methylpyrrolidin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat forebrain assessed as inhibition of [3H]histamine release from synaptosomes | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50237878 (2-(1,3-bis(3-(piperidin-1-yl)propyl)imidazolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat forebrain assessed as inhibition of [3H]histamine release from synaptosomes | Bioorg Med Chem Lett 18: 2288-91 (2008) Article DOI: 10.1016/j.bmcl.2008.03.006 BindingDB Entry DOI: 10.7270/Q2KW5FSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||