 Found 133 hits of Enzyme Inhibition Constant Data
Found 133 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313283

(1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...)Show SMILES Fc1ccc2c(c[nH]c2c1)C1=CCN(CCN2c3cccc4CCCN(c34)S2(=O)=O)CC1 |t:12| Show InChI InChI=1S/C24H25FN4O2S/c25-19-6-7-20-21(16-26-22(20)15-19)17-8-11-27(12-9-17)13-14-28-23-5-1-3-18-4-2-10-29(24(18)23)32(28,30)31/h1,3,5-8,15-16,26H,2,4,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313284

((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...)Show InChI InChI=1S/C14H18FNO2/c15-13-4-5-14(12-3-1-2-11(12)13)18-9-10-8-16-6-7-17-10/h4-5,10,16H,1-3,6-9H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5-HT2A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313272
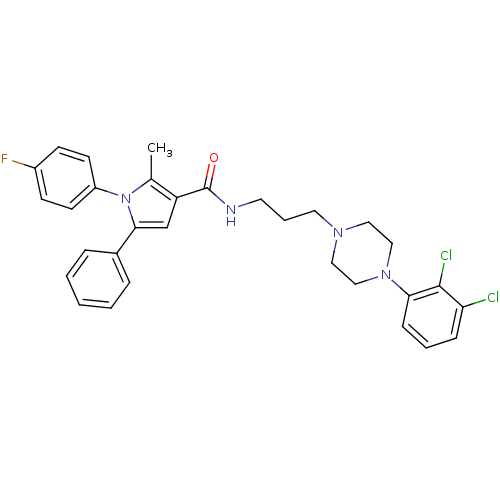
(CHEMBL1088314 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C31H31Cl2FN4O/c1-22-26(21-29(23-7-3-2-4-8-23)38(22)25-13-11-24(34)12-14-25)31(39)35-15-6-16-36-17-19-37(20-18-36)28-10-5-9-27(32)30(28)33/h2-5,7-14,21H,6,15-20H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313264

(CHEMBL1087773 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C33H37FN4O/c1-24-9-7-12-31(25(24)2)37-21-19-36(20-22-37)18-8-17-35-33(39)30-23-32(27-10-5-4-6-11-27)38(26(30)3)29-15-13-28(34)14-16-29/h4-7,9-16,23H,8,17-22H2,1-3H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313280

(CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C28H36N4O/c1-21-10-9-13-27(22(21)2)32-18-16-31(17-19-32)15-8-7-14-29-28(33)25-20-26(30-23(25)3)24-11-5-4-6-12-24/h4-6,9-13,20,30H,7-8,14-19H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313250

(CHEMBL1080726 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C26H31ClN4O/c1-20-24(19-25(29(20)2)21-8-4-3-5-9-21)26(32)28-12-7-13-30-14-16-31(17-15-30)23-11-6-10-22(27)18-23/h3-6,8-11,18-19H,7,12-17H2,1-2H3,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313259
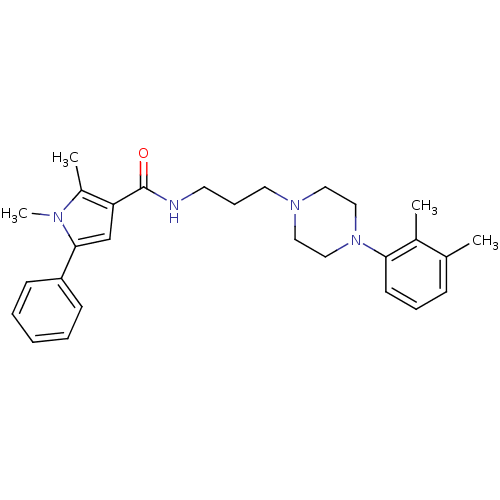
(CHEMBL1081644 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C28H36N4O/c1-21-10-8-13-26(22(21)2)32-18-16-31(17-19-32)15-9-14-29-28(33)25-20-27(30(4)23(25)3)24-11-6-5-7-12-24/h5-8,10-13,20H,9,14-19H2,1-4H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313248
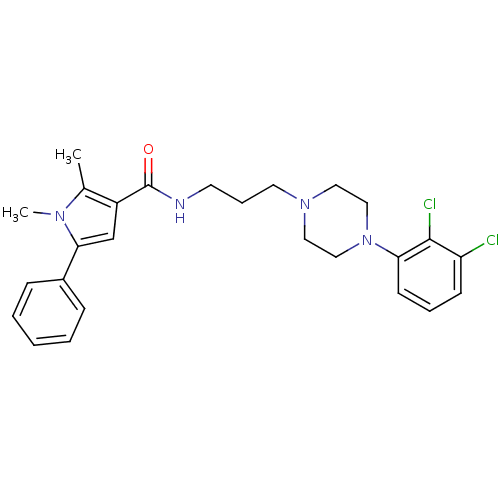
(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313265
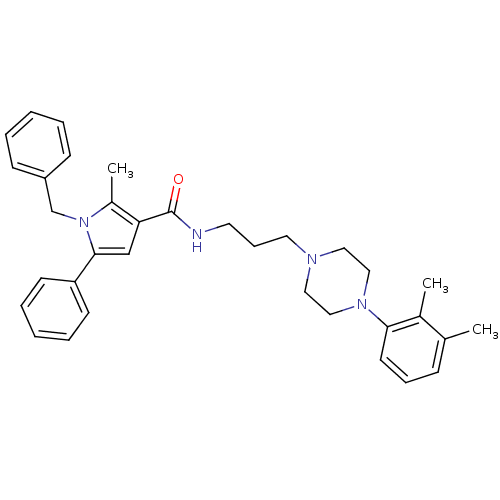
(1-benzyl-N-(3-(4-(2,3-dimethylphenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H40N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h4-10,12-17,24H,11,18-23,25H2,1-3H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313279
(CHEMBL1080711 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-3-15-35-22(2)24(21-27(35)23-10-5-4-6-11-23)29(36)32-14-7-8-16-33-17-19-34(20-18-33)26-13-9-12-25(30)28(26)31/h4-6,9-13,21H,3,7-8,14-20H2,1-2H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313280

(CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C28H36N4O/c1-21-10-9-13-27(22(21)2)32-18-16-31(17-19-32)15-8-7-14-29-28(33)25-20-26(30-23(25)3)24-11-5-4-6-12-24/h4-6,9-13,20,30H,7-8,14-19H2,1-3H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313255

(CHEMBL1080747 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C31H32ClFN4O/c1-23-29(22-30(24-7-3-2-4-8-24)37(23)27-13-11-26(33)12-14-27)31(38)34-15-6-16-35-17-19-36(20-18-35)28-10-5-9-25(32)21-28/h2-5,7-14,21-22H,6,15-20H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313251

(CHEMBL1080727 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C27H33ClN4O/c1-3-32-21(2)25(20-26(32)22-9-5-4-6-10-22)27(33)29-13-8-14-30-15-17-31(18-16-30)24-12-7-11-23(28)19-24/h4-7,9-12,19-20H,3,8,13-18H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313278
(CHEMBL1081036 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-20-22(19-25(31(20)2)21-9-4-3-5-10-21)27(34)30-13-6-7-14-32-15-17-33(18-16-32)24-12-8-11-23(28)26(24)29/h3-5,8-12,19H,6-7,13-18H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313278

(CHEMBL1081036 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-20-22(19-25(31(20)2)21-9-4-3-5-10-21)27(34)30-13-6-7-14-32-15-17-33(18-16-32)24-12-8-11-23(28)26(24)29/h3-5,8-12,19H,6-7,13-18H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313277
(CHEMBL1081035 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-13-33-20(2)22(19-25(33)21-8-5-4-6-9-21)27(34)30-12-14-31-15-17-32(18-16-31)24-11-7-10-23(28)26(24)29/h4-11,19H,3,12-18H2,1-2H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313260
(CHEMBL1086613 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-5-33-24(4)26(21-28(33)25-12-7-6-8-13-25)29(34)30-15-10-16-31-17-19-32(20-18-31)27-14-9-11-22(2)23(27)3/h6-9,11-14,21H,5,10,15-20H2,1-4H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313275

(CHEMBL1080522 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C24H26Cl2N4O/c1-17-19(16-21(28-17)18-6-3-2-4-7-18)24(31)27-10-11-29-12-14-30(15-13-29)22-9-5-8-20(25)23(22)26/h2-9,16,28H,10-15H2,1H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313275

(CHEMBL1080522 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C24H26Cl2N4O/c1-17-19(16-21(28-17)18-6-3-2-4-7-18)24(31)27-10-11-29-12-14-30(15-13-29)22-9-5-8-20(25)23(22)26/h2-9,16,28H,10-15H2,1H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313264

(CHEMBL1087773 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C33H37FN4O/c1-24-9-7-12-31(25(24)2)37-21-19-36(20-22-37)18-8-17-35-33(39)30-23-32(27-10-5-4-6-11-27)38(26(30)3)29-15-13-28(34)14-16-29/h4-7,9-16,23H,8,17-22H2,1-3H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313276

(CHEMBL1080683 | N-(2-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-23(29(18)2)19-7-4-3-5-8-19)25(32)28-11-12-30-13-15-31(16-14-30)22-10-6-9-21(26)24(22)27/h3-10,17H,11-16H2,1-2H3,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313259

(CHEMBL1081644 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C28H36N4O/c1-21-10-8-13-26(22(21)2)32-18-16-31(17-19-32)15-9-14-29-28(33)25-20-27(30(4)23(25)3)24-11-6-5-7-12-24/h5-8,10-13,20H,9,14-19H2,1-4H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313281

(CHEMBL1080554 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-22-11-10-14-27(23(22)2)33-19-17-32(18-20-33)16-9-8-15-30-29(34)26-21-28(31(4)24(26)3)25-12-6-5-7-13-25/h5-7,10-14,21H,8-9,15-20H2,1-4H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313281

(CHEMBL1080554 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-22-11-10-14-27(23(22)2)33-19-17-32(18-20-33)16-9-8-15-30-29(34)26-21-28(31(4)24(26)3)25-12-6-5-7-13-25/h5-7,10-14,21H,8-9,15-20H2,1-4H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313280

(CHEMBL1080712 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C28H36N4O/c1-21-10-9-13-27(22(21)2)32-18-16-31(17-19-32)15-8-7-14-29-28(33)25-20-26(30-23(25)3)24-11-5-4-6-12-24/h4-6,9-13,20,30H,7-8,14-19H2,1-3H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313268

(CHEMBL1086755 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-33-20(2)22(19-25(33)21-9-5-4-6-10-21)27(34)30-13-8-14-31-15-17-32(18-16-31)24-12-7-11-23(28)26(24)29/h4-7,9-12,19H,3,8,13-18H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313271
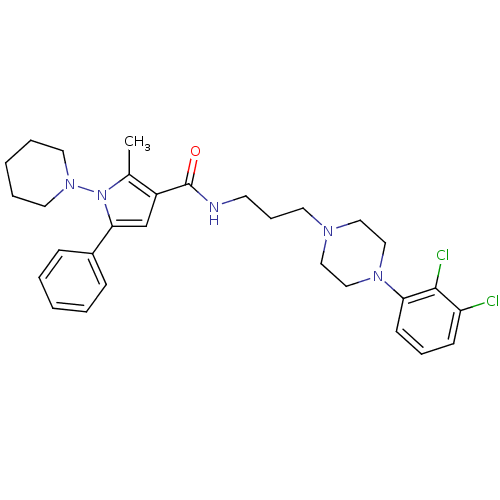
(CHEMBL1088313 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C30H37Cl2N5O/c1-23-25(22-28(24-10-4-2-5-11-24)37(23)36-16-6-3-7-17-36)30(38)33-14-9-15-34-18-20-35(21-19-34)27-13-8-12-26(31)29(27)32/h2,4-5,8,10-13,22H,3,6-7,9,14-21H2,1H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313260

(CHEMBL1086613 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-5-33-24(4)26(21-28(33)25-12-7-6-8-13-25)29(34)30-15-10-16-31-17-19-32(20-18-31)27-14-9-11-22(2)23(27)3/h6-9,11-14,21H,5,10,15-20H2,1-4H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313282

(CHEMBL1080555 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-5-17-35-26(4)28(23-30(35)27-13-7-6-8-14-27)31(36)32-16-9-10-18-33-19-21-34(22-20-33)29-15-11-12-24(2)25(29)3/h6-8,11-15,23H,5,9-10,16-22H2,1-4H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313261

(CHEMBL1088163 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C30H40N4O/c1-5-16-34-25(4)27(22-29(34)26-12-7-6-8-13-26)30(35)31-15-10-17-32-18-20-33(21-19-32)28-14-9-11-23(2)24(28)3/h6-9,11-14,22H,5,10,15-21H2,1-4H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313279

(CHEMBL1080711 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-3-15-35-22(2)24(21-27(35)23-10-5-4-6-11-23)29(36)32-14-7-8-16-33-17-19-34(20-18-33)26-13-9-12-25(30)28(26)31/h4-6,9-13,21H,3,7-8,14-20H2,1-2H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313258

(CHEMBL1081468 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C27H34N4O/c1-20-9-7-12-26(21(20)2)31-17-15-30(16-18-31)14-8-13-28-27(32)24-19-25(29-22(24)3)23-10-5-4-6-11-23/h4-7,9-12,19,29H,8,13-18H2,1-3H3,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313255

(CHEMBL1080747 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C31H32ClFN4O/c1-23-29(22-30(24-7-3-2-4-8-24)37(23)27-13-11-26(33)12-14-27)31(38)34-15-6-16-35-17-19-36(20-18-35)28-10-5-9-25(32)21-28/h2-5,7-14,21-22H,6,15-20H2,1H3,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313275

(CHEMBL1080522 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C24H26Cl2N4O/c1-17-19(16-21(28-17)18-6-3-2-4-7-18)24(31)27-10-11-29-12-14-30(15-13-29)22-9-5-8-20(25)23(22)26/h2-9,16,28H,10-15H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313250

(CHEMBL1080726 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C26H31ClN4O/c1-20-24(19-25(29(20)2)21-8-4-3-5-9-21)26(32)28-12-7-13-30-14-16-31(17-15-30)23-11-6-10-22(27)18-23/h3-6,8-11,18-19H,7,12-17H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313263

(CHEMBL1088165 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C32H43N5O/c1-25-12-10-15-30(26(25)2)35-22-20-34(21-23-35)17-11-16-33-32(38)29-24-31(28-13-6-4-7-14-28)37(27(29)3)36-18-8-5-9-19-36/h4,6-7,10,12-15,24H,5,8-9,11,16-23H2,1-3H3,(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313251

(CHEMBL1080727 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C27H33ClN4O/c1-3-32-21(2)25(20-26(32)22-9-5-4-6-10-22)27(33)29-13-8-14-30-15-17-31(18-16-30)24-12-7-11-23(28)19-24/h4-7,9-12,19-20H,3,8,13-18H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313249
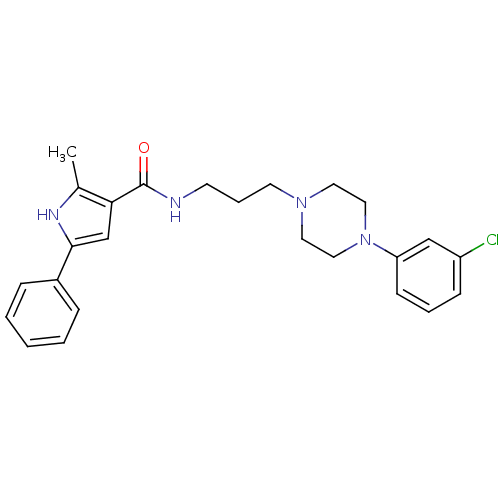
(CHEMBL1080215 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1)-c1ccccc1 Show InChI InChI=1S/C25H29ClN4O/c1-19-23(18-24(28-19)20-7-3-2-4-8-20)25(31)27-11-6-12-29-13-15-30(16-14-29)22-10-5-9-21(26)17-22/h2-5,7-10,17-18,28H,6,11-16H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313278

(CHEMBL1081036 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-20-22(19-25(31(20)2)21-9-4-3-5-10-21)27(34)30-13-6-7-14-32-15-17-33(18-16-32)24-12-8-11-23(28)26(24)29/h3-5,8-12,19H,6-7,13-18H2,1-2H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313252

(CHEMBL1080728 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H35ClN4O/c1-3-14-33-22(2)26(21-27(33)23-9-5-4-6-10-23)28(34)30-13-8-15-31-16-18-32(19-17-31)25-12-7-11-24(29)20-25/h4-7,9-12,20-21H,3,8,13-19H2,1-2H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50313269
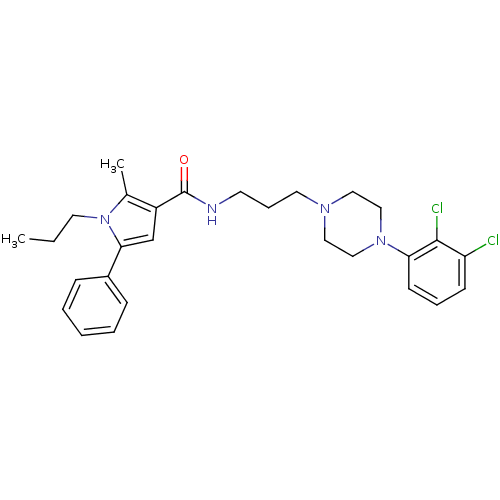
(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313279

(CHEMBL1080711 | N-(4-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-3-15-35-22(2)24(21-27(35)23-10-5-4-6-11-23)29(36)32-14-7-8-16-33-17-19-34(20-18-33)26-13-9-12-25(30)28(26)31/h4-6,9-13,21H,3,7-8,14-20H2,1-2H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313254

(CHEMBL1080746 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C30H38ClN5O/c1-24-28(23-29(25-10-4-2-5-11-25)36(24)35-16-6-3-7-17-35)30(37)32-14-9-15-33-18-20-34(21-19-33)27-13-8-12-26(31)22-27/h2,4-5,8,10-13,22-23H,3,6-7,9,14-21H2,1H3,(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313253

(CHEMBL1080745 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C29H37ClN4O/c1-22(2)21-34-23(3)27(20-28(34)24-9-5-4-6-10-24)29(35)31-13-8-14-32-15-17-33(18-16-32)26-12-7-11-25(30)19-26/h4-7,9-12,19-20,22H,8,13-18,21H2,1-3H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT1A receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313273

(1-benzyl-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H34Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h2-8,10-15,22H,9,16-21,23H2,1H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313261

(CHEMBL1088163 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C30H40N4O/c1-5-16-34-25(4)27(22-29(34)26-12-7-6-8-13-26)30(35)31-15-10-17-32-18-20-33(21-19-32)28-14-9-11-23(2)24(28)3/h6-9,11-14,22H,5,10,15-21H2,1-4H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313259

(CHEMBL1081644 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C28H36N4O/c1-21-10-8-13-26(22(21)2)32-18-16-31(17-19-32)15-9-14-29-28(33)25-20-27(30(4)23(25)3)24-11-6-5-7-12-24/h5-8,10-13,20H,9,14-19H2,1-4H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313263

(CHEMBL1088165 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C32H43N5O/c1-25-12-10-15-30(26(25)2)35-22-20-34(21-23-35)17-11-16-33-32(38)29-24-31(28-13-6-4-7-14-28)37(27(29)3)36-18-8-5-9-19-36/h4,6-7,10,12-15,24H,5,8-9,11,16-23H2,1-3H3,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313260

(CHEMBL1086613 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-5-33-24(4)26(21-28(33)25-12-7-6-8-13-25)29(34)30-15-10-16-31-17-19-32(20-18-31)27-14-9-11-22(2)23(27)3/h6-9,11-14,21H,5,10,15-20H2,1-4H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313281

(CHEMBL1080554 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C29H38N4O/c1-22-11-10-14-27(23(22)2)33-19-17-32(18-20-33)16-9-8-15-30-29(34)26-21-28(31(4)24(26)3)25-12-6-5-7-13-25/h5-7,10-14,21H,8-9,15-20H2,1-4H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313282

(CHEMBL1080555 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-5-17-35-26(4)28(23-30(35)27-13-7-6-8-14-27)31(36)32-16-9-10-18-33-19-21-34(22-20-33)29-15-11-12-24(2)25(29)3/h6-8,11-15,23H,5,9-10,16-22H2,1-4H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313276

(CHEMBL1080683 | N-(2-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-23(29(18)2)19-7-4-3-5-8-19)25(32)28-11-12-30-13-15-31(16-14-30)22-10-6-9-21(26)24(22)27/h3-10,17H,11-16H2,1-2H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313250

(CHEMBL1080726 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C26H31ClN4O/c1-20-24(19-25(29(20)2)21-8-4-3-5-9-21)26(32)28-12-7-13-30-14-16-31(17-15-30)23-11-6-10-22(27)18-23/h3-6,8-11,18-19H,7,12-17H2,1-2H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313264

(CHEMBL1087773 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C33H37FN4O/c1-24-9-7-12-31(25(24)2)37-21-19-36(20-22-37)18-8-17-35-33(39)30-23-32(27-10-5-4-6-11-27)38(26(30)3)29-15-13-28(34)14-16-29/h4-7,9-16,23H,8,17-22H2,1-3H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313276

(CHEMBL1080683 | N-(2-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-23(29(18)2)19-7-4-3-5-8-19)25(32)28-11-12-30-13-15-31(16-14-30)22-10-6-9-21(26)24(22)27/h3-10,17H,11-16H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313256
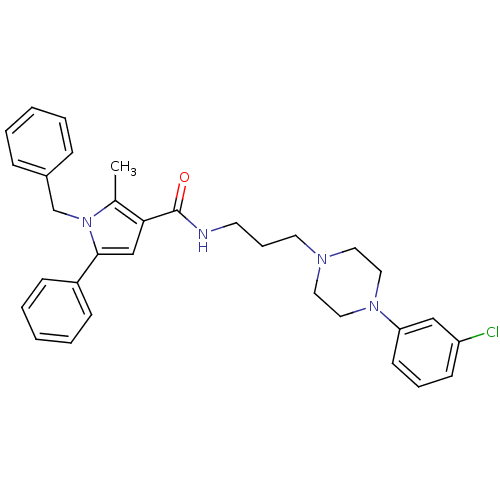
(1-benzyl-N-(3-(4-(3-chlorophenyl)piperazin-1-yl)pr...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H35ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h2-8,10-15,22-23H,9,16-21,24H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313268

(CHEMBL1086755 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-33-20(2)22(19-25(33)21-9-5-4-6-10-21)27(34)30-13-8-14-31-15-17-32(18-16-31)24-12-7-11-23(28)26(24)29/h4-7,9-12,19H,3,8,13-18H2,1-2H3,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313252

(CHEMBL1080728 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H35ClN4O/c1-3-14-33-22(2)26(21-27(33)23-9-5-4-6-10-23)28(34)30-13-8-15-31-16-18-32(19-17-31)25-12-7-11-24(29)20-25/h4-7,9-12,20-21H,3,8,13-19H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313251

(CHEMBL1080727 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C27H33ClN4O/c1-3-32-21(2)25(20-26(32)22-9-5-4-6-10-22)27(33)29-13-8-14-30-15-17-31(18-16-30)24-12-7-11-23(28)19-24/h4-7,9-12,19-20H,3,8,13-18H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313257

(CHEMBL1081303 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H41ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h3,6-8,12-15,22-23,26H,2,4-5,9-11,16-21,24H2,1H3,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313273

(1-benzyl-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H34Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h2-8,10-15,22H,9,16-21,23H2,1H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313265

(1-benzyl-N-(3-(4-(2,3-dimethylphenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H40N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h4-10,12-17,24H,11,18-23,25H2,1-3H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313261

(CHEMBL1088163 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C30H40N4O/c1-5-16-34-25(4)27(22-29(34)26-12-7-6-8-13-26)30(35)31-15-10-17-32-18-20-33(21-19-32)28-14-9-11-23(2)24(28)3/h6-9,11-14,22H,5,10,15-21H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313256

(1-benzyl-N-(3-(4-(3-chlorophenyl)piperazin-1-yl)pr...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H35ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h2-8,10-15,22-23H,9,16-21,24H2,1H3,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313249

(CHEMBL1080215 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1)-c1ccccc1 Show InChI InChI=1S/C25H29ClN4O/c1-19-23(18-24(28-19)20-7-3-2-4-8-20)25(31)27-11-6-12-29-13-15-30(16-14-29)22-10-5-9-21(26)17-22/h2-5,7-10,17-18,28H,6,11-16H2,1H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313272

(CHEMBL1088314 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C31H31Cl2FN4O/c1-22-26(21-29(23-7-3-2-4-8-23)38(22)25-13-11-24(34)12-14-25)31(39)35-15-6-16-36-17-19-37(20-18-36)28-10-5-9-27(32)30(28)33/h2-5,7-14,21H,6,15-20H2,1H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313252

(CHEMBL1080728 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H35ClN4O/c1-3-14-33-22(2)26(21-27(33)23-9-5-4-6-10-23)28(34)30-13-8-15-31-16-18-32(19-17-31)25-12-7-11-24(29)20-25/h4-7,9-12,20-21H,3,8,13-19H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313249

(CHEMBL1080215 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1)-c1ccccc1 Show InChI InChI=1S/C25H29ClN4O/c1-19-23(18-24(28-19)20-7-3-2-4-8-20)25(31)27-11-6-12-29-13-15-30(16-14-29)22-10-5-9-21(26)17-22/h2-5,7-10,17-18,28H,6,11-16H2,1H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313265

(1-benzyl-N-(3-(4-(2,3-dimethylphenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H40N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h4-10,12-17,24H,11,18-23,25H2,1-3H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313271

(CHEMBL1088313 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C30H37Cl2N5O/c1-23-25(22-28(24-10-4-2-5-11-24)37(23)36-16-6-3-7-17-36)30(38)33-14-9-15-34-18-20-35(21-19-34)27-13-8-12-26(31)29(27)32/h2,4-5,8,10-13,22H,3,6-7,9,14-21H2,1H3,(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313258

(CHEMBL1081468 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C27H34N4O/c1-20-9-7-12-26(21(20)2)31-17-15-30(16-18-31)14-8-13-28-27(32)24-19-25(29-22(24)3)23-10-5-4-6-11-23/h4-7,9-12,19,29H,8,13-18H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313277

(CHEMBL1081035 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-13-33-20(2)22(19-25(33)21-8-5-4-6-9-21)27(34)30-12-14-31-15-17-32(18-16-31)24-11-7-10-23(28)26(24)29/h4-11,19H,3,12-18H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313253

(CHEMBL1080745 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C29H37ClN4O/c1-22(2)21-34-23(3)27(20-28(34)24-9-5-4-6-10-24)29(35)31-13-8-14-32-15-17-33(18-16-32)26-12-7-11-25(30)19-26/h4-7,9-12,19-20,22H,8,13-18,21H2,1-3H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D3 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313270

(CHEMBL1088312 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-21(2)20-35-22(3)24(19-27(35)23-9-5-4-6-10-23)29(36)32-13-8-14-33-15-17-34(18-16-33)26-12-7-11-25(30)28(26)31/h4-7,9-12,19,21H,8,13-18,20H2,1-3H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313262

(CHEMBL1088164 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-23(2)22-35-26(5)28(21-30(35)27-12-7-6-8-13-27)31(36)32-15-10-16-33-17-19-34(20-18-33)29-14-9-11-24(3)25(29)4/h6-9,11-14,21,23H,10,15-20,22H2,1-5H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313266

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dimethylphenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H46N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h5,8-10,12,15-17,24,29H,4,6-7,11,13-14,18-23,25H2,1-3H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313273

(1-benzyl-N-(3-(4-(2,3-dichlorophenyl)piperazin-1-y...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H34Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h2-8,10-15,22H,9,16-21,23H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 354 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313268

(CHEMBL1086755 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-33-20(2)22(19-25(33)21-9-5-4-6-10-21)27(34)30-13-8-14-31-15-17-32(18-16-31)24-12-7-11-23(28)26(24)29/h4-7,9-12,19H,3,8,13-18H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313270

(CHEMBL1088312 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-21(2)20-35-22(3)24(19-27(35)23-9-5-4-6-10-23)29(36)32-13-8-14-33-15-17-34(18-16-33)26-12-7-11-25(30)28(26)31/h4-7,9-12,19,21H,8,13-18,20H2,1-3H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 381 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313270

(CHEMBL1088312 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C29H36Cl2N4O/c1-21(2)20-35-22(3)24(19-27(35)23-9-5-4-6-10-23)29(36)32-13-8-14-33-15-17-34(18-16-33)26-12-7-11-25(30)28(26)31/h4-7,9-12,19,21H,8,13-18,20H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313277

(CHEMBL1081035 | N-(2-(4-(2,3-dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C27H32Cl2N4O/c1-3-13-33-20(2)22(19-25(33)21-8-5-4-6-9-21)27(34)30-12-14-31-15-17-32(18-16-31)24-11-7-10-23(28)26(24)29/h4-11,19H,3,12-18H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313274

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dichlorophenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H40Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h3,6-8,12-15,22,25H,2,4-5,9-11,16-21,23H2,1H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313263

(CHEMBL1088165 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C32H43N5O/c1-25-12-10-15-30(26(25)2)35-22-20-34(21-23-35)17-11-16-33-32(38)29-24-31(28-13-6-4-7-14-28)37(27(29)3)36-18-8-5-9-19-36/h4,6-7,10,12-15,24H,5,8-9,11,16-23H2,1-3H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human SERT expressed in HEK293 cells after 30 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313254

(CHEMBL1080746 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C30H38ClN5O/c1-24-28(23-29(25-10-4-2-5-11-25)36(24)35-16-6-3-7-17-35)30(37)32-14-9-15-33-18-20-34(21-19-33)27-13-8-12-26(31)22-27/h2,4-5,8,10-13,22-23H,3,6-7,9,14-21H2,1H3,(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313255

(CHEMBL1080747 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C31H32ClFN4O/c1-23-29(22-30(24-7-3-2-4-8-24)37(23)27-13-11-26(33)12-14-27)31(38)34-15-6-16-35-17-19-36(20-18-35)28-10-5-9-25(32)21-28/h2-5,7-14,21-22H,6,15-20H2,1H3,(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313254

(CHEMBL1080746 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C30H38ClN5O/c1-24-28(23-29(25-10-4-2-5-11-25)36(24)35-16-6-3-7-17-35)30(37)32-14-9-15-33-18-20-34(21-19-33)27-13-8-12-26(31)22-27/h2,4-5,8,10-13,22-23H,3,6-7,9,14-21H2,1H3,(H,32,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313272

(CHEMBL1088314 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C31H31Cl2FN4O/c1-22-26(21-29(23-7-3-2-4-8-23)38(22)25-13-11-24(34)12-14-25)31(39)35-15-6-16-36-17-19-37(20-18-36)28-10-5-9-27(32)30(28)33/h2-5,7-14,21H,6,15-20H2,1H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 466 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50313271

(CHEMBL1088313 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C30H37Cl2N5O/c1-23-25(22-28(24-10-4-2-5-11-24)37(23)36-16-6-3-7-17-36)30(38)33-14-9-15-34-18-20-35(21-19-34)27-13-8-12-26(31)29(27)32/h2,4-5,8,10-13,22H,3,6-7,9,14-21H2,1H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50313272

(CHEMBL1088314 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1-c1ccc(F)cc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C31H31Cl2FN4O/c1-22-26(21-29(23-7-3-2-4-8-23)38(22)25-13-11-24(34)12-14-25)31(39)35-15-6-16-36-17-19-37(20-18-36)28-10-5-9-27(32)30(28)33/h2-5,7-14,21H,6,15-20H2,1H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 599 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313257

(CHEMBL1081303 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H41ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h3,6-8,12-15,22-23,26H,2,4-5,9-11,16-21,24H2,1H3,(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 611 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313258

(CHEMBL1081468 | N-(3-(4-(2,3-Dimethylphenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C)-c1ccccc1 Show InChI InChI=1S/C27H34N4O/c1-20-9-7-12-26(21(20)2)31-17-15-30(16-18-31)14-8-13-28-27(32)24-19-25(29-22(24)3)23-10-5-4-6-11-23/h4-7,9-12,19,29H,8,13-18H2,1-3H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 615 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313266

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dimethylphenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H46N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h5,8-10,12,15-17,24,29H,4,6-7,11,13-14,18-23,25H2,1-3H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 638 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D4 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 713 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT6 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313282

(CHEMBL1080555 | N-(4-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-5-17-35-26(4)28(23-30(35)27-13-7-6-8-14-27)31(36)32-16-9-10-18-33-19-21-34(22-20-33)29-15-11-12-24(2)25(29)3/h6-8,11-15,23H,5,9-10,16-22H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 792 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313271

(CHEMBL1088313 | N-(3-(4-(2,3-dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1N1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C30H37Cl2N5O/c1-23-25(22-28(24-10-4-2-5-11-24)37(23)36-16-6-3-7-17-36)30(38)33-14-9-15-34-18-20-35(21-19-34)27-13-8-12-26(31)29(27)32/h2,4-5,8,10-13,22H,3,6-7,9,14-21H2,1H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 795 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313262

(CHEMBL1088164 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-23(2)22-35-26(5)28(21-30(35)27-12-7-6-8-13-27)31(36)32-15-10-16-33-17-19-34(20-18-33)29-14-9-11-24(3)25(29)4/h6-9,11-14,21,23H,10,15-20,22H2,1-5H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 897 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D3 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 984 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D4 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313274

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dichlorophenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H40Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h3,6-8,12-15,22,25H,2,4-5,9-11,16-21,23H2,1H3,(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313257

(CHEMBL1081303 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H41ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h3,6-8,12-15,22-23,26H,2,4-5,9-11,16-21,24H2,1H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D3 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313266

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dimethylphenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C34H46N4O/c1-26-12-10-17-32(27(26)2)37-22-20-36(21-23-37)19-11-18-35-34(39)31-24-33(30-15-8-5-9-16-30)38(28(31)3)25-29-13-6-4-7-14-29/h5,8-10,12,15-17,24,29H,4,6-7,11,13-14,18-23,25H2,1-3H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313262

(CHEMBL1088164 | N-(3-(4-(2,3-dimethylphenyl)pipera...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(C)c1C Show InChI InChI=1S/C31H42N4O/c1-23(2)22-35-26(5)28(21-30(35)27-12-7-6-8-13-27)31(36)32-15-10-16-33-17-19-34(20-18-33)29-14-9-11-24(3)25(29)4/h6-9,11-14,21,23H,10,15-20,22H2,1-5H3,(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT6 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313256

(1-benzyl-N-(3-(4-(3-chlorophenyl)piperazin-1-yl)pr...)Show SMILES Cc1c(cc(-c2ccccc2)n1Cc1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H35ClN4O/c1-25-30(23-31(27-12-6-3-7-13-27)37(25)24-26-10-4-2-5-11-26)32(38)34-16-9-17-35-18-20-36(21-19-35)29-15-8-14-28(33)22-29/h2-8,10-15,22-23H,9,16-21,24H2,1H3,(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50313253

(CHEMBL1080745 | N-(3-(4-(3-chlorophenyl)piperazin-...)Show SMILES CC(C)Cn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C29H37ClN4O/c1-22(2)21-34-23(3)27(20-28(34)24-9-5-4-6-10-24)29(35)31-13-8-14-32-15-17-33(18-16-32)26-12-7-11-25(30)19-26/h4-7,9-12,19-20,22H,8,13-18,21H2,1-3H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human recombinant 5-HT2C receptor expressed in CHO-K1 cells after 60 mins by rapid filtration assay |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50313267

(CHEMBL1086626 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1[nH]c(cc1C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl)-c1ccccc1 Show InChI InChI=1S/C25H28Cl2N4O/c1-18-20(17-22(29-18)19-7-3-2-4-8-19)25(32)28-11-6-12-30-13-15-31(16-14-30)23-10-5-9-21(26)24(23)27/h2-5,7-10,17,29H,6,11-16H2,1H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D4 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT6 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50313269

(CHEMBL1086756 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES CCCn1c(C)c(cc1-c1ccccc1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C28H34Cl2N4O/c1-3-14-34-21(2)23(20-26(34)22-9-5-4-6-10-22)28(35)31-13-8-15-32-16-18-33(19-17-32)25-12-7-11-24(29)27(25)30/h4-7,9-12,20H,3,8,13-19H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 receptor |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50313274

(1-(cyclohexylmethyl)-N-(3-(4-(2,3-dichlorophenyl)p...)Show SMILES Cc1c(cc(-c2ccccc2)n1CC1CCCCC1)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C32H40Cl2N4O/c1-24-27(22-30(26-12-6-3-7-13-26)38(24)23-25-10-4-2-5-11-25)32(39)35-16-9-17-36-18-20-37(21-19-36)29-15-8-14-28(33)31(29)34/h3,6-8,12-15,22,25H,2,4-5,9-11,16-21,23H2,1H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor expressed in CHO-K1 cells after 60 mins |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50313248

(CHEMBL1086754 | N-(3-(4-(2,3-Dichlorophenyl)pipera...)Show SMILES Cc1c(cc(-c2ccccc2)n1C)C(=O)NCCCN1CCN(CC1)c1cccc(Cl)c1Cl Show InChI InChI=1S/C26H30Cl2N4O/c1-19-21(18-24(30(19)2)20-8-4-3-5-9-20)26(33)29-12-7-13-31-14-16-32(17-15-31)23-11-6-10-22(27)25(23)28/h3-6,8-11,18H,7,12-17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 1705-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.093
BindingDB Entry DOI: 10.7270/Q2SN093C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data