 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50011762
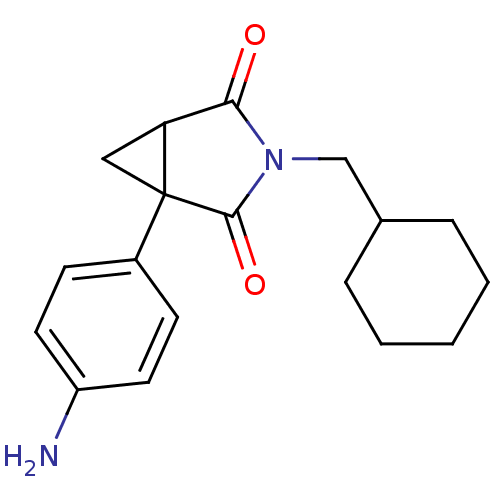
((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 Aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 Aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of estrogen production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011756
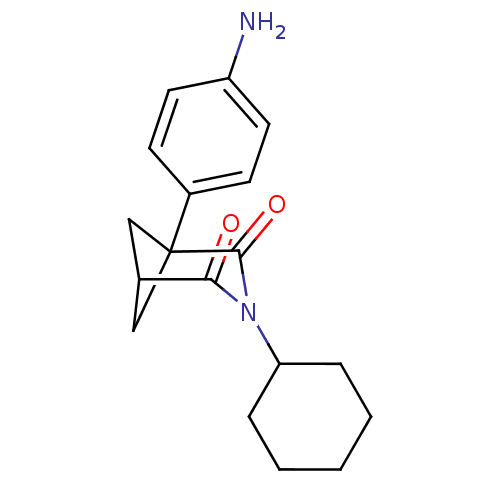
(1-(4-Amino-phenyl)-3-cyclohexyl-3-aza-bicyclo[3.1....)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)N(C1CCCCC1)C2=O |(8.24,.27,;6.91,-.5,;6.9,-2.04,;5.55,-2.81,;4.22,-2.02,;4.21,-.47,;5.55,.28,;2.88,-2.79,;1.55,-2.02,;.23,-2.79,;1.57,-3.56,;.23,-4.33,;-1.11,-5.11,;1.55,-5.11,;1.55,-6.65,;2.88,-7.4,;2.88,-8.96,;1.55,-9.71,;.22,-8.94,;.22,-7.4,;2.88,-4.33,;4.22,-5.11,)| Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-12(11-18)16(21)20(17(18)22)15-4-2-1-3-5-15/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011767
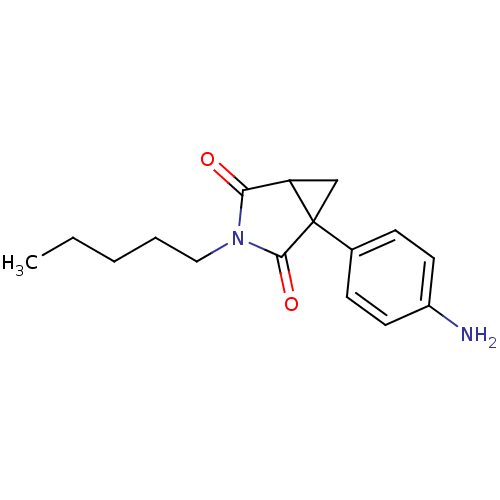
(1-(4-Amino-phenyl)-3-pentyl-3-aza-bicyclo[3.1.0]he...)Show InChI InChI=1S/C16H20N2O2/c1-2-3-4-9-18-14(19)13-10-16(13,15(18)20)11-5-7-12(17)8-6-11/h5-8,13H,2-4,9-10,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011766

(1-(4-Amino-phenyl)-3-pentyl-3-aza-bicyclo[3.1.1]he...)Show SMILES CCCCCN1C(=O)C2CC(C2)(C1=O)c1ccc(N)cc1 |(-1.11,-11.25,;-1.11,-9.71,;.22,-8.94,;.22,-7.4,;1.55,-6.63,;1.55,-5.09,;.23,-4.32,;-1.11,-5.09,;.23,-2.78,;1.55,-2.01,;2.88,-2.78,;1.56,-3.55,;2.88,-4.32,;4.21,-5.09,;4.21,-2.01,;5.54,-2.8,;6.88,-2.04,;6.89,-.5,;8.22,.27,;5.54,.28,;4.2,-.47,)| Show InChI InChI=1S/C17H22N2O2/c1-2-3-4-9-19-15(20)12-10-17(11-12,16(19)21)13-5-7-14(18)8-6-13/h5-8,12H,2-4,9-11,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011765
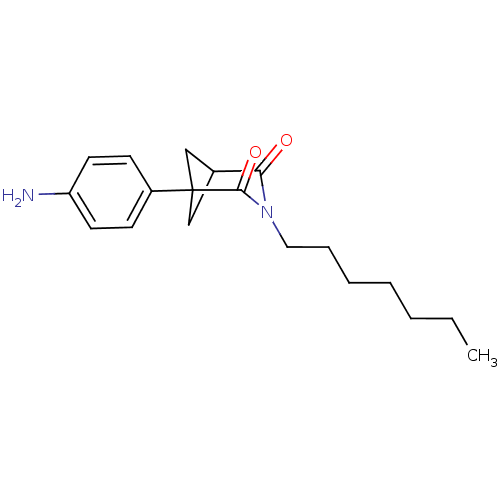
(1-(4-Amino-phenyl)-3-heptyl-3-aza-bicyclo[3.1.1]he...)Show SMILES CCCCCCCN1C(=O)C2CC(C2)(C1=O)c1ccc(N)cc1 |(-2.44,-13.56,;-2.44,-12.02,;-1.11,-11.25,;-1.11,-9.71,;.22,-8.94,;.22,-7.4,;1.55,-6.63,;1.55,-5.09,;.23,-4.32,;-1.11,-5.09,;.23,-2.78,;1.55,-2.01,;2.88,-2.78,;1.56,-3.55,;2.88,-4.32,;4.21,-5.09,;4.21,-2.01,;5.54,-2.8,;6.88,-2.04,;6.89,-.5,;8.22,.27,;5.54,.28,;4.2,-.47,)| Show InChI InChI=1S/C19H26N2O2/c1-2-3-4-5-6-11-21-17(22)14-12-19(13-14,18(21)23)15-7-9-16(20)10-8-15/h7-10,14H,2-6,11-13,20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011768
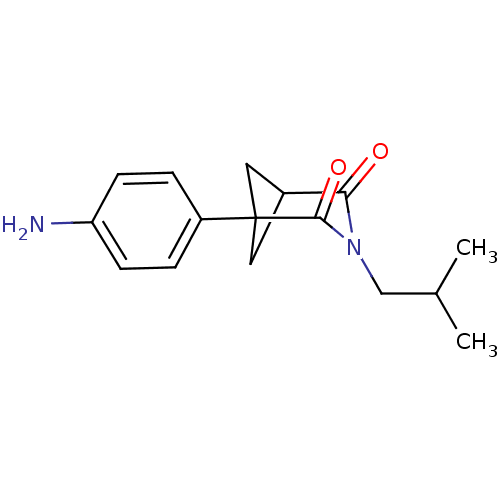
(1-(4-Amino-phenyl)-3-isobutyl-3-aza-bicyclo[3.1.1]...)Show SMILES CC(C)CN1C(=O)C2CC(C2)(C1=O)c1ccc(N)cc1 |(.22,-8.96,;.22,-7.42,;-1.11,-6.65,;1.55,-6.65,;1.55,-5.11,;.23,-4.33,;-1.11,-5.11,;.23,-2.79,;1.55,-2.02,;2.88,-2.79,;1.57,-3.56,;2.88,-4.33,;4.22,-5.11,;4.22,-2.02,;5.55,-2.81,;6.9,-2.04,;6.91,-.5,;8.24,.27,;5.55,.28,;4.21,-.47,)| Show InChI InChI=1S/C16H20N2O2/c1-10(2)9-18-14(19)11-7-16(8-11,15(18)20)12-3-5-13(17)6-4-12/h3-6,10-11H,7-9,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011756

(1-(4-Amino-phenyl)-3-cyclohexyl-3-aza-bicyclo[3.1....)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)N(C1CCCCC1)C2=O |(8.24,.27,;6.91,-.5,;6.9,-2.04,;5.55,-2.81,;4.22,-2.02,;4.21,-.47,;5.55,.28,;2.88,-2.79,;1.55,-2.02,;.23,-2.79,;1.57,-3.56,;.23,-4.33,;-1.11,-5.11,;1.55,-5.11,;1.55,-6.65,;2.88,-7.4,;2.88,-8.96,;1.55,-9.71,;.22,-8.94,;.22,-7.4,;2.88,-4.33,;4.22,-5.11,)| Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-12(11-18)16(21)20(17(18)22)15-4-2-1-3-5-15/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of estrogen production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011764
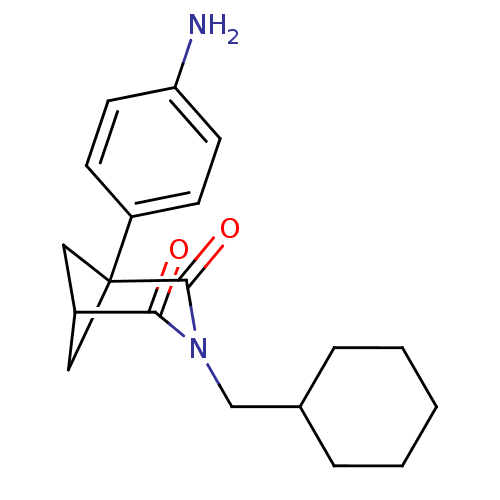
(1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza-bicycl...)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)N(CC1CCCCC1)C2=O |(8.24,.27,;6.91,-.5,;6.9,-2.04,;5.55,-2.81,;4.22,-2.02,;4.21,-.47,;5.55,.28,;2.88,-2.79,;1.55,-2.02,;.23,-2.79,;1.57,-3.56,;.23,-4.33,;-1.11,-5.11,;1.55,-5.11,;1.55,-6.65,;.22,-7.42,;.23,-8.96,;-1.11,-9.71,;-2.45,-8.94,;-2.45,-7.4,;-1.11,-6.64,;2.88,-4.33,;4.22,-5.11,)| Show InChI InChI=1S/C19H24N2O2/c20-16-8-6-15(7-9-16)19-10-14(11-19)17(22)21(18(19)23)12-13-4-2-1-3-5-13/h6-9,13-14H,1-5,10-12,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011761
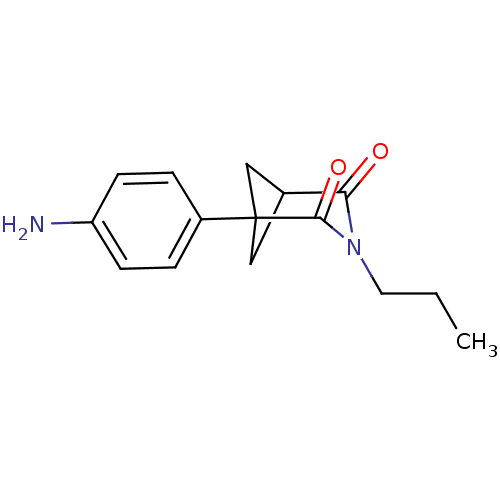
(1-(4-Amino-phenyl)-3-propyl-3-aza-bicyclo[3.1.1]he...)Show SMILES CCCN1C(=O)C2CC(C2)(C1=O)c1ccc(N)cc1 |(.22,-8.94,;.22,-7.4,;1.55,-6.63,;1.55,-5.09,;.23,-4.32,;-1.11,-5.09,;.23,-2.78,;1.55,-2.01,;2.88,-2.78,;1.56,-3.55,;2.88,-4.32,;4.21,-5.09,;4.21,-2.01,;5.54,-2.8,;6.88,-2.04,;6.89,-.5,;8.22,.27,;5.54,.28,;4.2,-.47,)| Show InChI InChI=1S/C15H18N2O2/c1-2-7-17-13(18)10-8-15(9-10,14(17)19)11-3-5-12(16)6-4-11/h3-6,10H,2,7-9,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011759
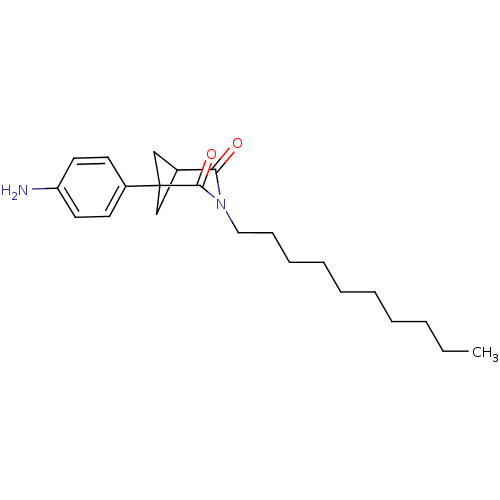
(1-(4-Amino-phenyl)-3-decyl-3-aza-bicyclo[3.1.1]hep...)Show SMILES CCCCCCCCCCN1C(=O)C2CC(C2)(C1=O)c1ccc(N)cc1 |(-5.11,-16.64,;-3.78,-15.87,;-3.78,-14.33,;-2.44,-13.56,;-2.44,-12.02,;-1.11,-11.25,;-1.11,-9.71,;.22,-8.94,;.22,-7.4,;1.55,-6.63,;1.55,-5.09,;.23,-4.32,;-1.11,-5.09,;.23,-2.78,;1.55,-2.01,;2.88,-2.78,;1.56,-3.55,;2.88,-4.32,;4.21,-5.09,;4.21,-2.01,;5.54,-2.8,;6.88,-2.04,;6.89,-.5,;8.22,.27,;5.54,.28,;4.2,-.47,)| Show InChI InChI=1S/C22H32N2O2/c1-2-3-4-5-6-7-8-9-14-24-20(25)17-15-22(16-17,21(24)26)18-10-12-19(23)13-11-18/h10-13,17H,2-9,14-16,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of progesterone production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011758

(1-(4-Amino-phenyl)-3-(4-methoxy-benzyl)-3-aza-bicy...)Show SMILES COc1ccc(CN2C(=O)C3CC(C3)(C2=O)c2ccc(N)cc2)cc1 |(-5.12,-8.95,;-3.78,-9.72,;-2.44,-8.93,;-2.44,-7.39,;-1.11,-6.62,;.22,-7.41,;1.55,-6.64,;1.55,-5.1,;.23,-4.33,;-1.11,-5.1,;.23,-2.79,;1.55,-2.02,;2.88,-2.79,;1.57,-3.56,;2.88,-4.33,;4.21,-5.1,;4.21,-2.02,;5.54,-2.8,;6.89,-2.04,;6.9,-.5,;8.23,.27,;5.54,.28,;4.2,-.47,;.23,-8.93,;-1.1,-9.7,)| Show InChI InChI=1S/C20H20N2O3/c1-25-17-8-2-13(3-9-17)12-22-18(23)14-10-20(11-14,19(22)24)15-4-6-16(21)7-5-15/h2-9,14H,10-12,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011763
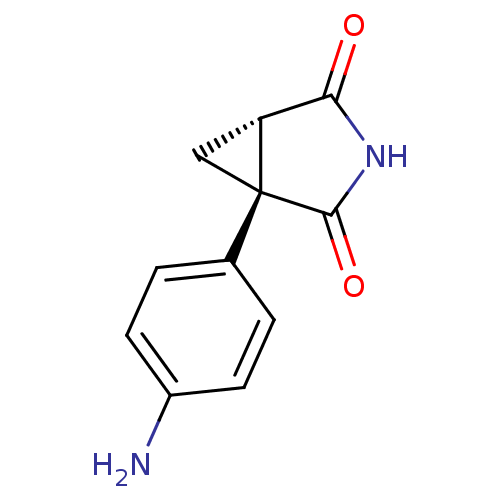
(1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...)Show InChI InChI=1S/C11H10N2O2/c12-7-3-1-6(2-4-7)11-5-8(11)9(14)13-10(11)15/h1-4,8H,5,12H2,(H,13,14,15)/t8-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011760
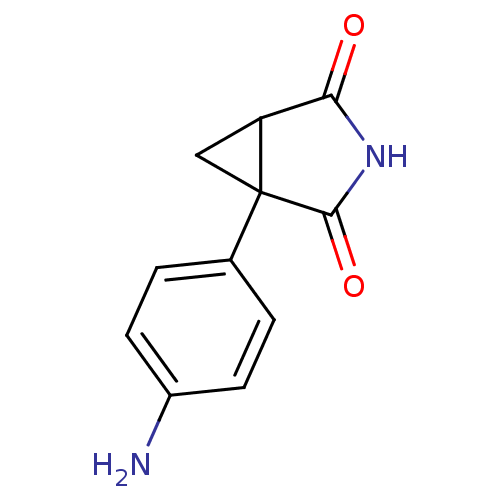
(1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...)Show InChI InChI=1S/C11H10N2O2/c12-7-3-1-6(2-4-7)11-5-8(11)9(14)13-10(11)15/h1-4,8H,5,12H2,(H,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A1 aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 Aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011755
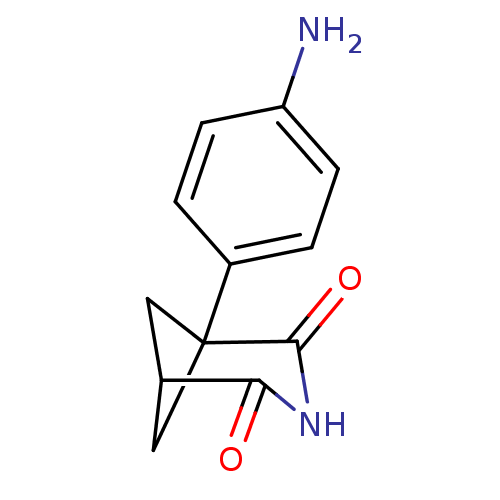
(1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.1]heptane-2,4...)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)NC2=O |(8.22,.27,;6.89,-.5,;5.54,.28,;4.2,-.47,;4.21,-2.01,;5.54,-2.8,;6.88,-2.04,;2.88,-2.78,;1.55,-2.01,;.23,-2.78,;1.56,-3.55,;.23,-4.32,;-1.11,-5.09,;1.55,-5.09,;2.88,-4.32,;4.21,-5.09,)| Show InChI InChI=1S/C12H12N2O2/c13-9-3-1-8(2-4-9)12-5-7(6-12)10(15)14-11(12)16/h1-4,7H,5-6,13H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human placental Cytochrome P450 19A aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldosterone production in rat adrenal tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of estrogen production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldosterone production in rat adrenal tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of progesterone production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldosterone production in rat adrenal tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50011756

(1-(4-Amino-phenyl)-3-cyclohexyl-3-aza-bicyclo[3.1....)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)N(C1CCCCC1)C2=O |(8.24,.27,;6.91,-.5,;6.9,-2.04,;5.55,-2.81,;4.22,-2.02,;4.21,-.47,;5.55,.28,;2.88,-2.79,;1.55,-2.02,;.23,-2.79,;1.57,-3.56,;.23,-4.33,;-1.11,-5.11,;1.55,-5.11,;1.55,-6.65,;2.88,-7.4,;2.88,-8.96,;1.55,-9.71,;.22,-8.94,;.22,-7.4,;2.88,-4.33,;4.22,-5.11,)| Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-12(11-18)16(21)20(17(18)22)15-4-2-1-3-5-15/h6-9,12,15H,1-5,10-11,19H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldosterone production in rat adrenal tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50011756

(1-(4-Amino-phenyl)-3-cyclohexyl-3-aza-bicyclo[3.1....)Show SMILES Nc1ccc(cc1)C12CC(C1)C(=O)N(C1CCCCC1)C2=O |(8.24,.27,;6.91,-.5,;6.9,-2.04,;5.55,-2.81,;4.22,-2.02,;4.21,-.47,;5.55,.28,;2.88,-2.79,;1.55,-2.02,;.23,-2.79,;1.57,-3.56,;.23,-4.33,;-1.11,-5.11,;1.55,-5.11,;1.55,-6.65,;2.88,-7.4,;2.88,-8.96,;1.55,-9.71,;.22,-8.94,;.22,-7.4,;2.88,-4.33,;4.22,-5.11,)| Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-12(11-18)16(21)20(17(18)22)15-4-2-1-3-5-15/h6-9,12,15H,1-5,10-11,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of progesterone production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of progesterone production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Rattus norvegicus) | BDBM50011762

((1R,5S)1-(4-Amino-phenyl)-3-cyclohexylmethyl-3-aza...)Show InChI InChI=1S/C18H22N2O2/c19-14-8-6-13(7-9-14)18-10-15(18)16(21)20(17(18)22)11-12-4-2-1-3-5-12/h6-9,12,15H,1-5,10-11,19H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of estrogen production in hamster ovarian tissue |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011757
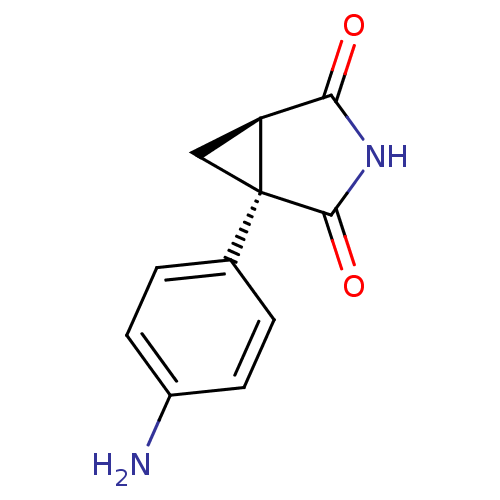
(1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...)Show InChI InChI=1S/C11H10N2O2/c12-7-3-1-6(2-4-7)11-5-8(11)9(14)13-10(11)15/h1-4,8H,5,12H2,(H,13,14,15)/t8-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG.
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 Aromatase |
J Med Chem 34: 1329-34 (1991)
BindingDB Entry DOI: 10.7270/Q2QN65RW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data