

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308280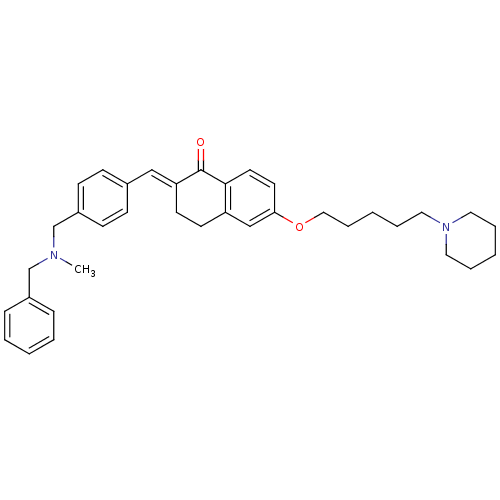 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE-mediated hydrolysis of acetylcholine by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949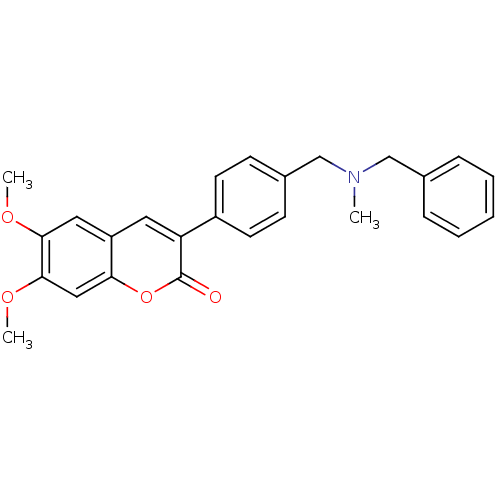 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308280 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308281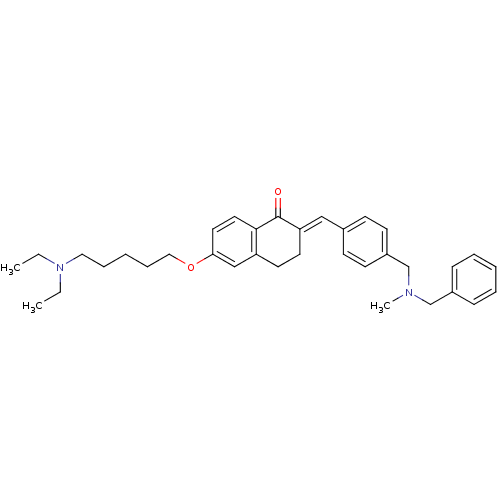 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308262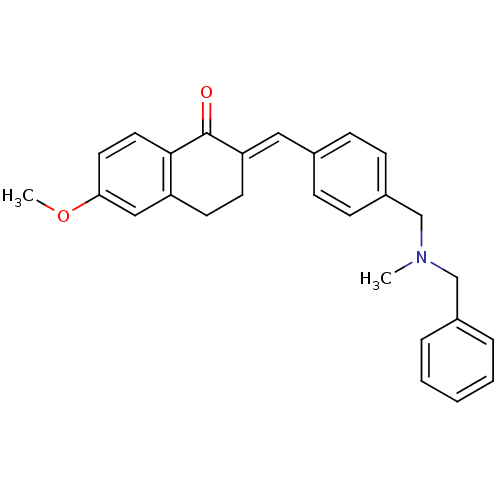 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308267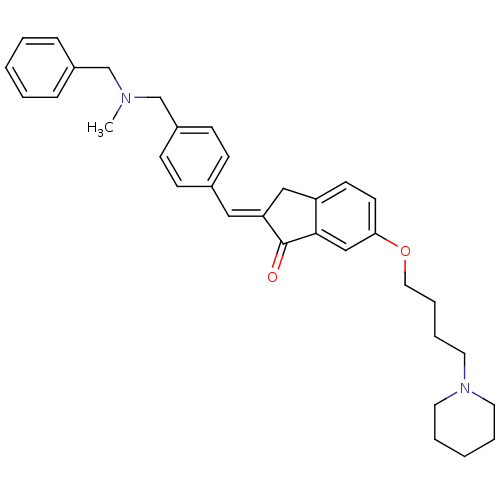 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308282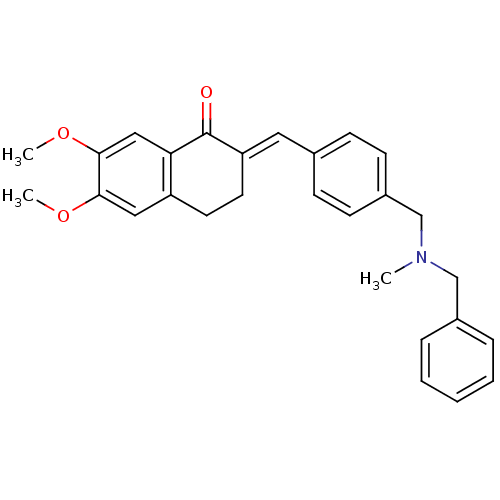 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6,7-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308266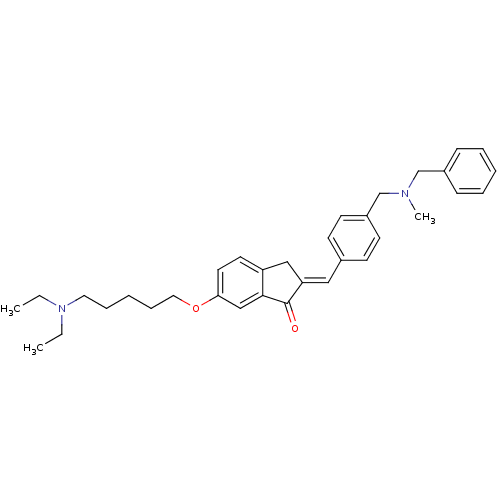 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308265 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308264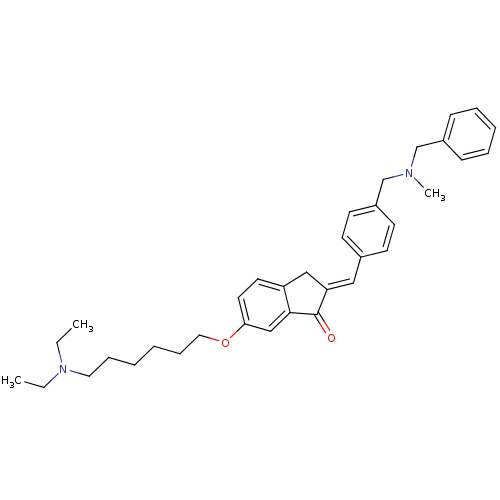 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308263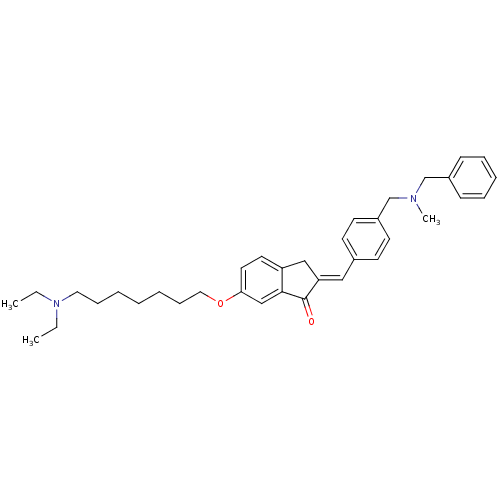 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308277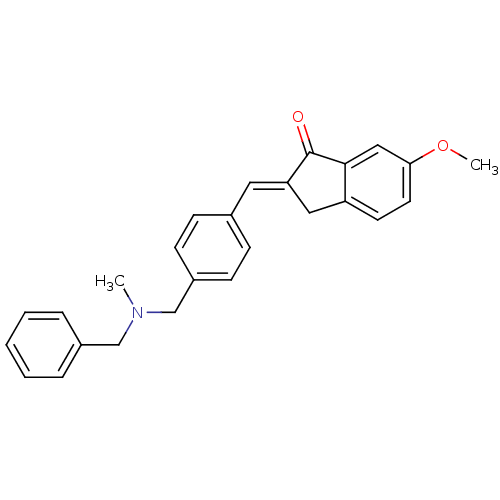 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308268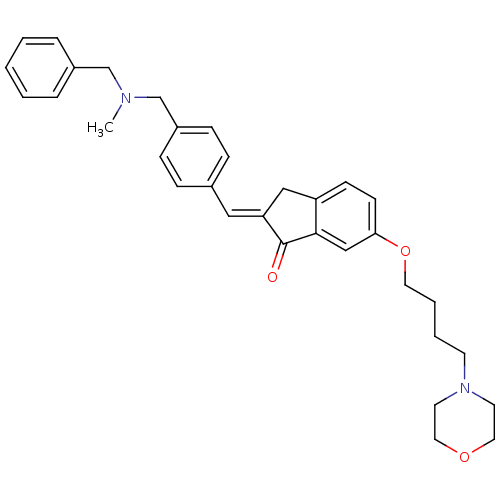 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308269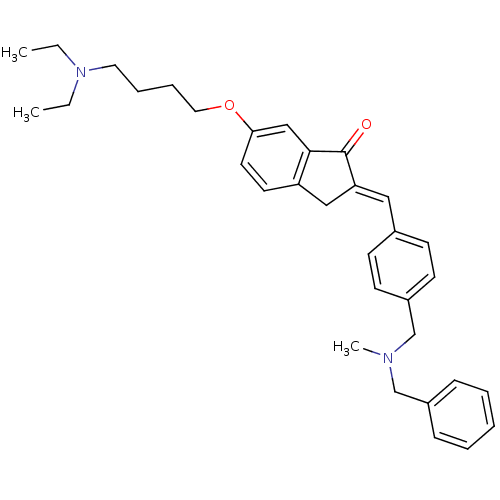 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308279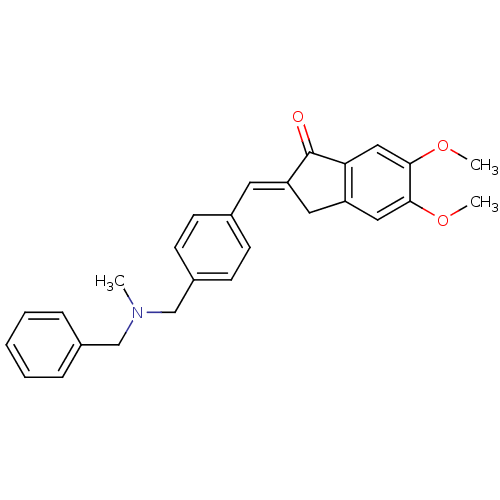 (CHEMBL598632 | N-(2-chloro-5-(S-2-fluoroethyl)thio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308270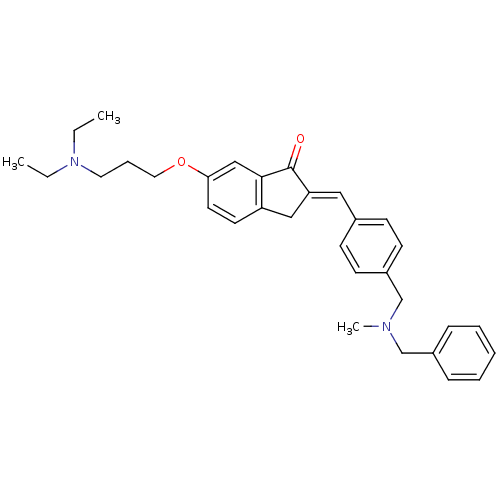 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308271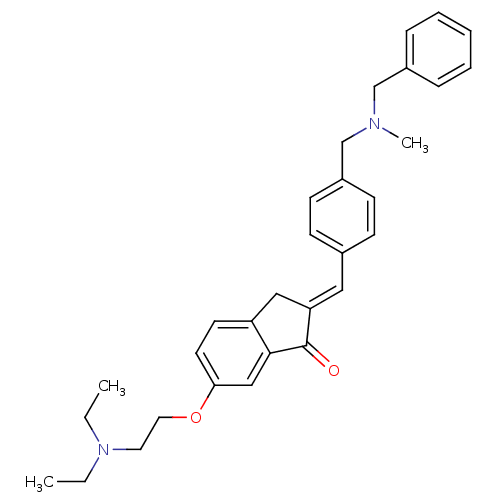 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308273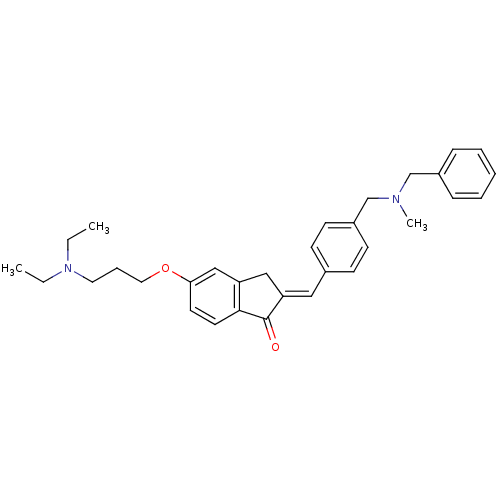 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308266 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308274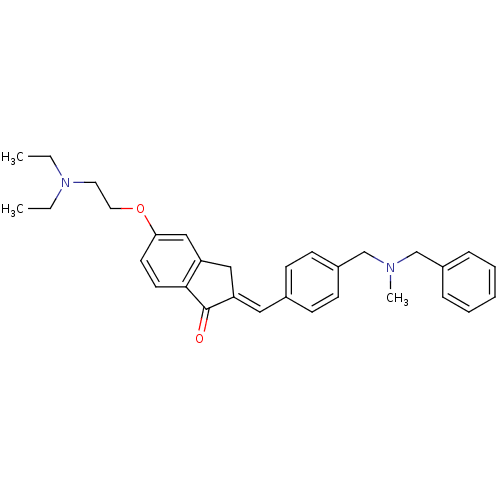 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308269 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308272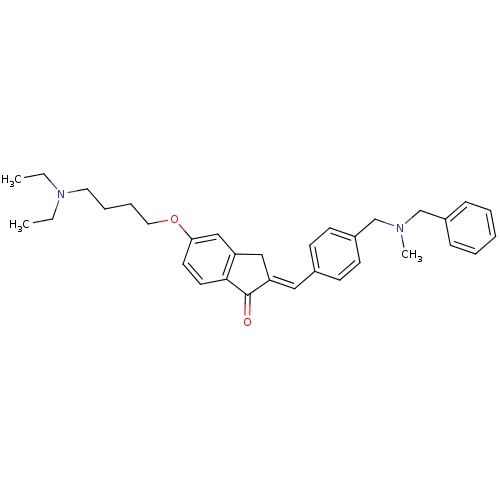 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308276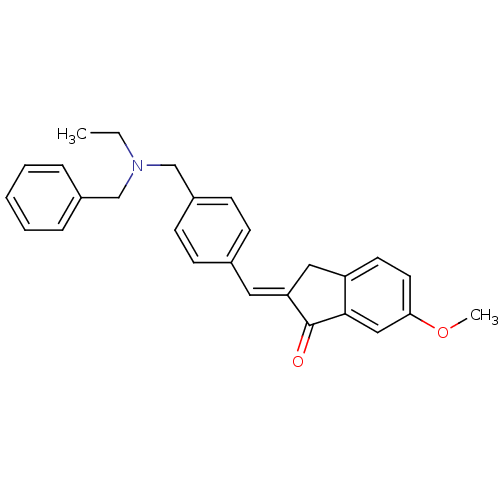 (2-{4-[(Benzylethylamino)methyl]benzylidene}-6-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308280 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308283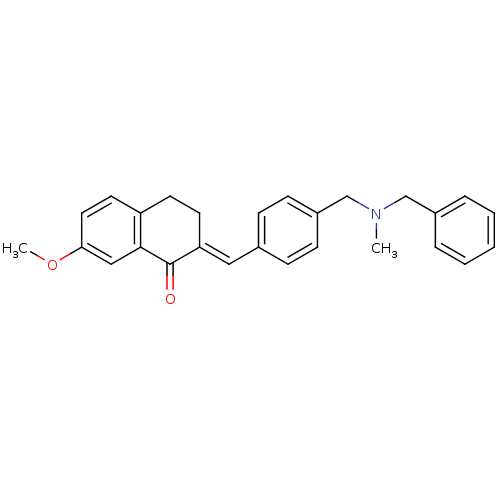 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-7-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308265 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308277 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308281 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308267 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308273 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308275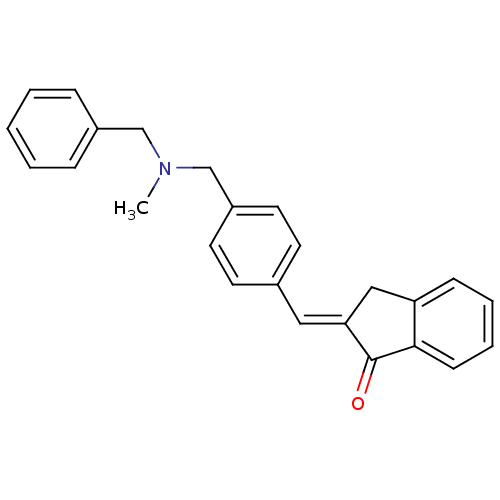 (2-{4-[(Benzylmethylamino)methyl]benzylidene}indan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308270 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308271 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308264 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308278 (2-Chloro-5-(S-2-fluoroethyl)thioaniline | CHEMBL61...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308274 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308272 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-5-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308278 (2-Chloro-5-(S-2-fluoroethyl)thioaniline | CHEMBL61...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308275 (2-{4-[(Benzylmethylamino)methyl]benzylidene}indan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308263 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10949 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308282 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308262 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308276 (2-{4-[(Benzylethylamino)methyl]benzylidene}-6-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308268 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308283 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-7-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308279 (CHEMBL598632 | N-(2-chloro-5-(S-2-fluoroethyl)thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human BuChE preincubated for 20 mins before substrate addition by Ellman's method | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||