 Found 110 hits of Enzyme Inhibition Constant Data
Found 110 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329315
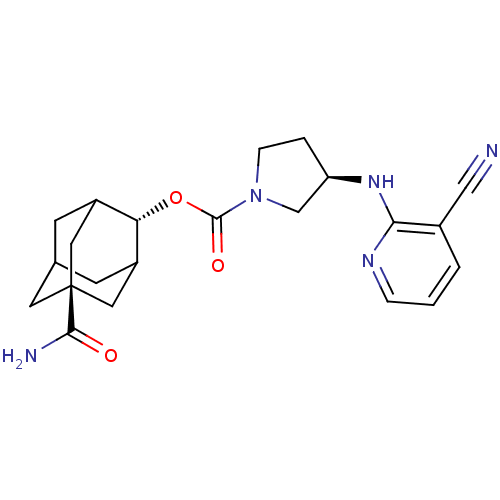
((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329313
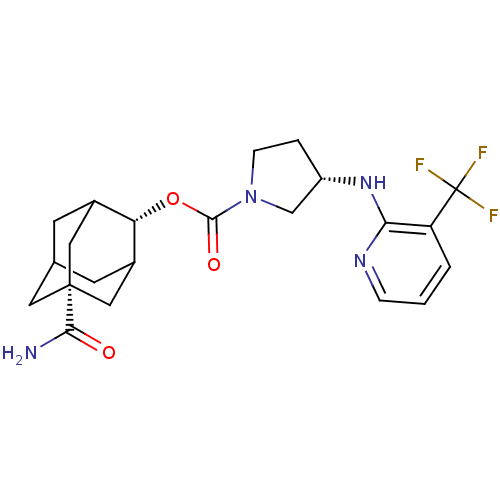
((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wD:16.19,3.2,9.10,TLB:30:29:4.5.6:8,THB:30:5:8:31.29.9,9:29:4:6.7.8,9:7:4:31.30.29,10:9:4.5.6:8,(23.92,-31.27,;25.27,-30.5,;25.27,-28.96,;26.61,-31.27,;25.41,-32.55,;26.91,-32.13,;26.91,-30.55,;27.95,-29.31,;26.6,-29.79,;29.34,-29.89,;30.68,-29.13,;32.01,-29.91,;32,-31.45,;33.35,-29.16,;33.38,-27.61,;34.85,-27.16,;35.74,-28.42,;34.81,-29.65,;37.28,-28.44,;38.07,-27.12,;37.32,-25.79,;38.1,-24.47,;39.65,-24.49,;40.4,-25.84,;39.61,-27.16,;40.35,-28.5,;39.56,-29.82,;41.89,-28.53,;41.11,-29.83,;29.33,-31.42,;28.32,-32.7,;27.94,-31.76,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329309
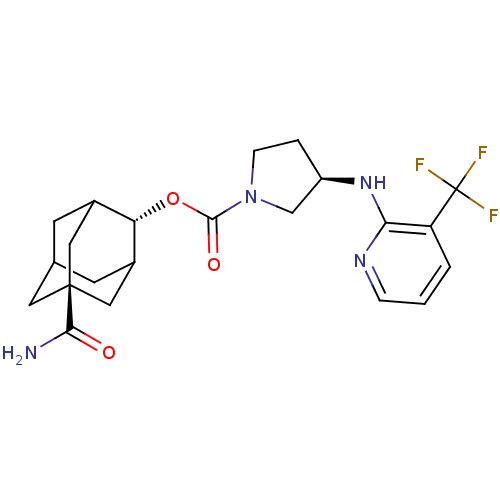
((R)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(7.67,-22.53,;9.01,-21.75,;9.02,-20.21,;10.35,-22.53,;9.16,-23.81,;10.66,-23.39,;12.06,-23.95,;13.08,-22.68,;11.68,-23.02,;13.09,-21.15,;14.43,-20.39,;15.75,-21.17,;15.74,-22.71,;17.1,-20.41,;17.12,-18.87,;18.6,-18.42,;19.48,-19.68,;18.55,-20.91,;21.02,-19.7,;21.81,-18.38,;21.06,-17.05,;21.85,-15.73,;23.39,-15.75,;24.14,-17.09,;23.35,-18.41,;24.1,-19.76,;23.3,-21.08,;25.64,-19.79,;24.86,-21.09,;11.69,-20.57,;10.65,-21.8,;10.35,-21.05,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329313

((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wD:16.19,3.2,9.10,TLB:30:29:4.5.6:8,THB:30:5:8:31.29.9,9:29:4:6.7.8,9:7:4:31.30.29,10:9:4.5.6:8,(23.92,-31.27,;25.27,-30.5,;25.27,-28.96,;26.61,-31.27,;25.41,-32.55,;26.91,-32.13,;26.91,-30.55,;27.95,-29.31,;26.6,-29.79,;29.34,-29.89,;30.68,-29.13,;32.01,-29.91,;32,-31.45,;33.35,-29.16,;33.38,-27.61,;34.85,-27.16,;35.74,-28.42,;34.81,-29.65,;37.28,-28.44,;38.07,-27.12,;37.32,-25.79,;38.1,-24.47,;39.65,-24.49,;40.4,-25.84,;39.61,-27.16,;40.35,-28.5,;39.56,-29.82,;41.89,-28.53,;41.11,-29.83,;29.33,-31.42,;28.32,-32.7,;27.94,-31.76,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329312
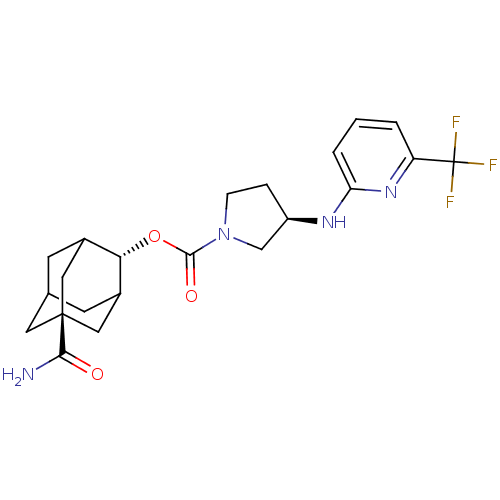
((R)-3-(6-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(4.49,-33.95,;5.83,-33.18,;5.84,-31.64,;7.17,-33.96,;5.98,-35.23,;7.48,-34.81,;8.88,-35.38,;9.9,-34.1,;8.5,-34.45,;9.91,-32.57,;11.25,-31.82,;12.57,-32.6,;12.56,-34.14,;13.92,-31.84,;13.94,-30.29,;15.41,-29.84,;16.3,-31.1,;15.37,-32.33,;17.84,-31.13,;18.63,-29.81,;20.17,-29.84,;20.96,-28.52,;20.21,-27.17,;18.66,-27.15,;17.88,-28.47,;17.91,-25.81,;18.7,-24.48,;16.37,-25.79,;17.13,-24.47,;8.51,-31.99,;7.47,-33.23,;7.16,-32.47,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-3-17(28-16)27-15-4-5-29(11-15)20(31)32-18-13-6-12-7-14(18)10-21(8-12,9-13)19(26)30/h1-3,12-15,18H,4-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329314
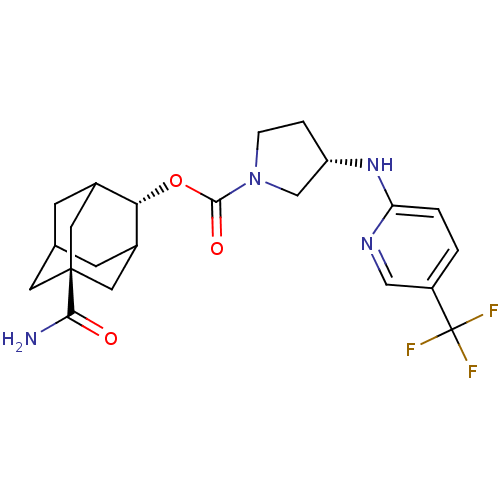
((S)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-11.32,-46.08,;-9.96,-45.31,;-9.95,-43.77,;-8.62,-46.08,;-9.81,-47.36,;-8.31,-46.94,;-6.91,-47.5,;-5.89,-46.23,;-7.29,-46.57,;-5.88,-44.7,;-4.54,-43.94,;-3.22,-44.72,;-3.23,-46.26,;-1.87,-43.97,;-1.85,-42.42,;-.37,-41.97,;.51,-43.23,;-.42,-44.46,;2.05,-43.25,;2.84,-41.93,;4.38,-41.96,;5.17,-40.64,;4.42,-39.3,;2.88,-39.28,;2.09,-40.6,;5.21,-37.98,;6.75,-38,;4.46,-36.63,;5.97,-36.63,;-7.28,-44.12,;-8.32,-45.36,;-8.62,-44.6,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329311

((R)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.14,-33.31,;-8.79,-32.53,;-8.79,-30.99,;-7.45,-33.31,;-8.65,-34.59,;-7.15,-34.17,;-5.74,-34.73,;-4.73,-33.46,;-6.12,-33.8,;-4.72,-31.93,;-3.37,-31.17,;-2.05,-31.95,;-2.06,-33.49,;-.71,-31.19,;-.68,-29.65,;.79,-29.2,;1.68,-30.46,;.75,-31.69,;3.22,-30.48,;4.01,-29.16,;5.55,-29.19,;6.34,-27.87,;5.59,-26.53,;4.04,-26.51,;3.26,-27.83,;6.38,-25.2,;7.92,-25.23,;5.63,-23.86,;7.14,-23.86,;-6.11,-31.35,;-7.15,-32.58,;-7.46,-31.83,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329309

((R)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(7.67,-22.53,;9.01,-21.75,;9.02,-20.21,;10.35,-22.53,;9.16,-23.81,;10.66,-23.39,;12.06,-23.95,;13.08,-22.68,;11.68,-23.02,;13.09,-21.15,;14.43,-20.39,;15.75,-21.17,;15.74,-22.71,;17.1,-20.41,;17.12,-18.87,;18.6,-18.42,;19.48,-19.68,;18.55,-20.91,;21.02,-19.7,;21.81,-18.38,;21.06,-17.05,;21.85,-15.73,;23.39,-15.75,;24.14,-17.09,;23.35,-18.41,;24.1,-19.76,;23.3,-21.08,;25.64,-19.79,;24.86,-21.09,;11.69,-20.57,;10.65,-21.8,;10.35,-21.05,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329312

((R)-3-(6-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(4.49,-33.95,;5.83,-33.18,;5.84,-31.64,;7.17,-33.96,;5.98,-35.23,;7.48,-34.81,;8.88,-35.38,;9.9,-34.1,;8.5,-34.45,;9.91,-32.57,;11.25,-31.82,;12.57,-32.6,;12.56,-34.14,;13.92,-31.84,;13.94,-30.29,;15.41,-29.84,;16.3,-31.1,;15.37,-32.33,;17.84,-31.13,;18.63,-29.81,;20.17,-29.84,;20.96,-28.52,;20.21,-27.17,;18.66,-27.15,;17.88,-28.47,;17.91,-25.81,;18.7,-24.48,;16.37,-25.79,;17.13,-24.47,;8.51,-31.99,;7.47,-33.23,;7.16,-32.47,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-3-17(28-16)27-15-4-5-29(11-15)20(31)32-18-13-6-12-7-14(18)10-21(8-12,9-13)19(26)30/h1-3,12-15,18H,4-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329316
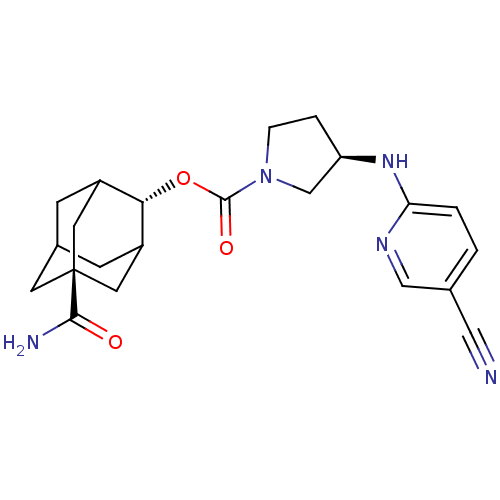
((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329318
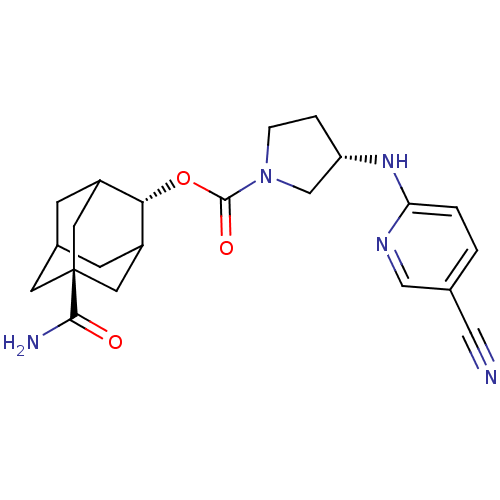
((S)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.94,-49.07,;25.29,-48.29,;25.29,-46.75,;26.62,-49.07,;25.43,-50.35,;26.93,-49.93,;28.33,-50.49,;29.35,-49.21,;27.95,-49.56,;29.36,-47.69,;30.7,-46.93,;32.03,-47.71,;32.01,-49.25,;33.37,-46.95,;33.39,-45.41,;34.87,-44.95,;35.75,-46.21,;34.83,-47.45,;37.29,-46.24,;38.08,-44.92,;39.62,-44.95,;40.41,-43.63,;39.67,-42.28,;38.12,-42.26,;37.33,-43.58,;40.45,-40.96,;41.24,-39.64,;27.96,-47.11,;26.92,-48.34,;26.62,-47.58,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329306
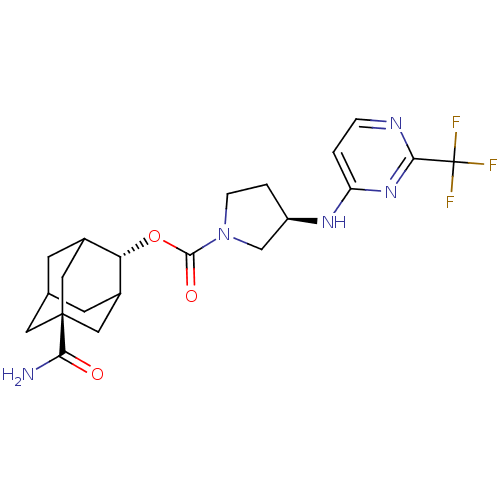
((R)-3-(2-Trifluoromethyl-pyrimidin-4-ylamino)-pyrr...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccnc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(6.77,-10.92,;8.12,-10.15,;8.12,-8.61,;9.46,-10.93,;8.26,-12.2,;9.76,-11.78,;11.17,-12.35,;12.18,-11.07,;10.79,-11.42,;12.19,-9.54,;13.53,-8.79,;14.86,-9.57,;14.85,-11.11,;16.2,-8.81,;16.23,-7.26,;17.7,-6.81,;18.59,-8.07,;17.66,-9.3,;20.13,-8.1,;20.92,-6.78,;22.46,-6.81,;23.25,-5.49,;22.5,-4.14,;20.95,-4.12,;20.17,-5.44,;20.2,-2.78,;20.99,-1.45,;18.66,-2.76,;19.41,-1.44,;10.8,-8.96,;9.76,-10.2,;9.45,-9.44,)| Show InChI InChI=1S/C21H26F3N5O3/c22-21(23,24)18-26-3-1-15(28-18)27-14-2-4-29(10-14)19(31)32-16-12-5-11-6-13(16)9-20(7-11,8-12)17(25)30/h1,3,11-14,16H,2,4-10H2,(H2,25,30)(H,26,27,28)/t11?,12?,13?,14-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329292
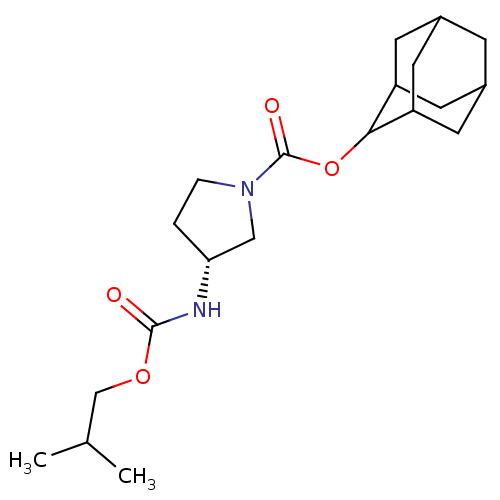
((R)-3-Isobutoxycarbonylamino-pyrrolidine-1-carboxy...)Show SMILES CC(C)COC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(5.77,-14.44,;4.98,-15.76,;5.73,-17.11,;3.44,-15.74,;2.65,-17.06,;1.11,-17.04,;.36,-15.69,;.32,-18.36,;-1.22,-18.33,;-2.11,-17.07,;-3.58,-17.52,;-3.61,-19.07,;-2.15,-19.56,;-4.95,-19.83,;-4.96,-21.37,;-6.28,-19.04,;-7.62,-19.8,;-7.63,-21.33,;-9.02,-21.68,;-10.35,-21.19,;-11.55,-22.46,;-10.05,-22.04,;-8.64,-22.61,;-10.05,-20.46,;-9.01,-19.22,;-10.36,-19.7,)| Show InChI InChI=1S/C20H32N2O4/c1-12(2)11-25-19(23)21-17-3-4-22(10-17)20(24)26-18-15-6-13-5-14(8-15)9-16(18)7-13/h12-18H,3-11H2,1-2H3,(H,21,23)/t13?,14?,15?,16?,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader in presenc... |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329317
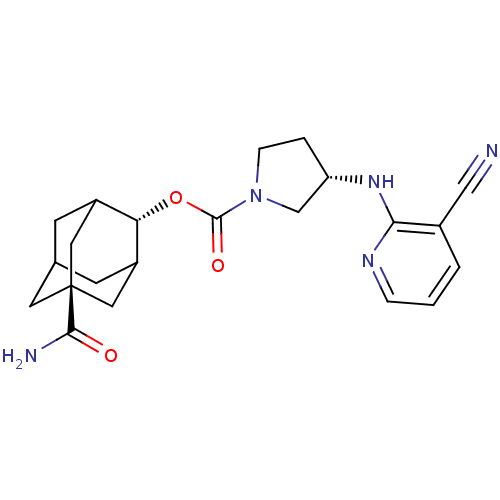
((S)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(2.39,-49.17,;3.73,-48.39,;3.74,-46.85,;5.07,-49.17,;3.88,-50.45,;5.38,-50.03,;6.78,-50.59,;7.8,-49.31,;6.4,-49.66,;7.81,-47.79,;9.15,-47.03,;10.47,-47.81,;10.46,-49.35,;11.82,-47.05,;11.84,-45.51,;13.31,-45.06,;14.2,-46.32,;13.27,-47.55,;15.74,-46.34,;16.53,-45.02,;15.78,-43.69,;16.56,-42.36,;18.11,-42.39,;18.86,-43.73,;18.07,-45.05,;18.82,-46.4,;19.56,-47.74,;6.41,-47.21,;5.37,-48.44,;5.06,-47.69,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329314

((S)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-11.32,-46.08,;-9.96,-45.31,;-9.95,-43.77,;-8.62,-46.08,;-9.81,-47.36,;-8.31,-46.94,;-6.91,-47.5,;-5.89,-46.23,;-7.29,-46.57,;-5.88,-44.7,;-4.54,-43.94,;-3.22,-44.72,;-3.23,-46.26,;-1.87,-43.97,;-1.85,-42.42,;-.37,-41.97,;.51,-43.23,;-.42,-44.46,;2.05,-43.25,;2.84,-41.93,;4.38,-41.96,;5.17,-40.64,;4.42,-39.3,;2.88,-39.28,;2.09,-40.6,;5.21,-37.98,;6.75,-38,;4.46,-36.63,;5.97,-36.63,;-7.28,-44.12,;-8.32,-45.36,;-8.62,-44.6,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329288
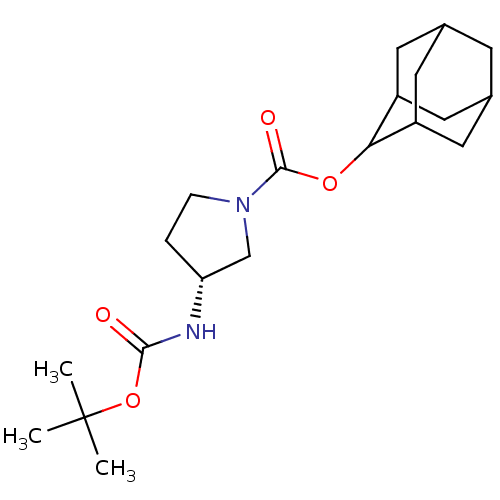
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(33.72,4.21,;32.18,4.23,;31.44,5.58,;32.95,5.58,;31.39,2.91,;29.85,2.94,;29.11,4.28,;29.06,1.62,;27.52,1.64,;26.64,2.9,;25.17,2.45,;25.14,.9,;26.6,.41,;23.8,.15,;23.78,-1.39,;22.47,.93,;21.13,.17,;21.12,-1.36,;19.72,-1.7,;18.4,-1.21,;17.2,-2.49,;18.7,-2.07,;20.11,-2.63,;18.7,-.49,;19.73,.75,;18.39,.27,)| Show InChI InChI=1S/C20H32N2O4/c1-20(2,3)26-18(23)21-16-4-5-22(11-16)19(24)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329295
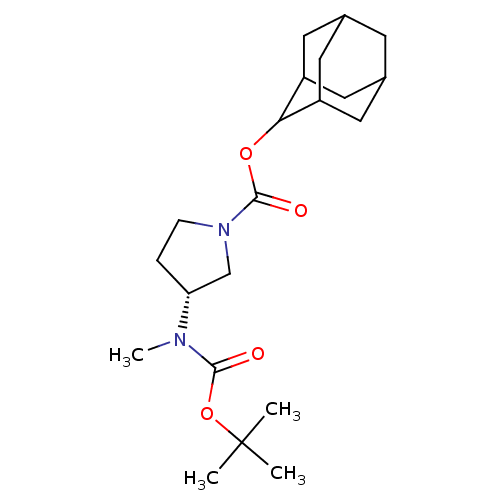
((R)-3-(tert-Butoxycarbonyl-methyl-amino)-pyrrolidi...)Show SMILES CN([C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |r,wU:2.1,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:10:11:14:17.18.19,10:18:14:12.16.11,9:10:14.15.17:19,(2.39,-29.14,;1.64,-27.79,;.1,-27.77,;-.79,-26.51,;-2.26,-26.96,;-2.29,-28.5,;-.83,-29,;-3.63,-29.26,;-3.64,-30.8,;-4.95,-28.48,;-6.29,-29.24,;-6.31,-30.76,;-7.7,-31.11,;-9.03,-30.62,;-10.23,-31.9,;-8.72,-31.48,;-7.32,-32.04,;-8.73,-29.89,;-7.69,-28.66,;-9.04,-29.14,;2.43,-26.47,;1.68,-25.12,;3.97,-26.49,;4.76,-25.17,;6.3,-25.2,;4.01,-23.83,;5.52,-23.83,)| Show InChI InChI=1S/C21H34N2O4/c1-21(2,3)27-19(24)22(4)17-5-6-23(12-17)20(25)26-18-15-8-13-7-14(10-15)11-16(18)9-13/h13-18H,5-12H2,1-4H3/t13?,14?,15?,16?,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329310

((R)-3-(4-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cc(ccn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(25.09,-21.61,;26.44,-20.84,;26.44,-19.3,;27.78,-21.61,;26.58,-22.89,;28.08,-22.47,;29.49,-23.04,;30.5,-21.76,;29.1,-22.1,;30.51,-20.23,;31.85,-19.47,;33.18,-20.25,;33.16,-21.79,;34.52,-19.5,;34.55,-17.95,;36.02,-17.5,;36.9,-18.76,;35.98,-19.99,;38.44,-18.78,;39.23,-17.46,;40.77,-17.5,;41.56,-16.18,;40.82,-14.83,;39.27,-14.81,;38.48,-16.13,;43.1,-16.2,;43.85,-17.55,;43.9,-14.88,;44.64,-16.2,;29.11,-19.65,;28.08,-20.89,;27.77,-20.13,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-3-27-17(7-15)28-16-2-4-29(11-16)20(31)32-18-13-5-12-6-14(18)10-21(8-12,9-13)19(26)30/h1,3,7,12-14,16,18H,2,4-6,8-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader in presenc... |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329302
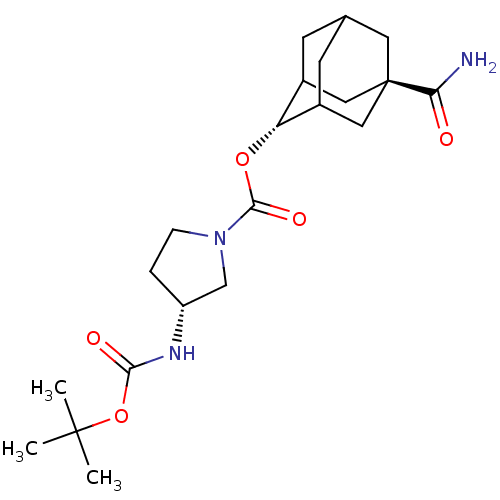
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329297
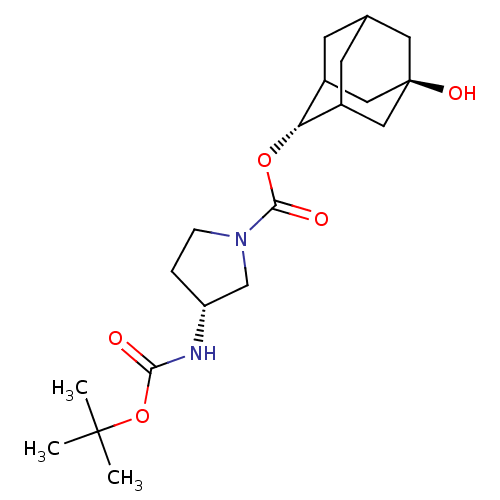
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:8.7,16.16,wD:23.25,TLB:16:17:25:20.21.22,15:16:25.19.20:22,THB:18:19:22:26.17.16,18:17:25.19.20:22,16:21:25:26.18.17,(6.15,-33.79,;4.61,-33.76,;3.86,-32.41,;5.37,-32.42,;3.82,-35.08,;2.28,-35.06,;1.53,-33.71,;1.49,-36.38,;-.05,-36.35,;-.93,-35.09,;-2.41,-35.54,;-2.43,-37.09,;-.98,-37.58,;-3.78,-37.85,;-3.79,-39.39,;-5.1,-37.07,;-6.44,-37.82,;-6.45,-39.35,;-7.47,-40.63,;-8.87,-40.06,;-8.88,-38.48,;-7.84,-37.24,;-9.18,-37.72,;-9.18,-39.21,;-10.52,-38.43,;-10.37,-40.48,;-7.85,-39.7,)| Show InChI InChI=1S/C20H32N2O5/c1-19(2,3)27-17(23)21-15-4-5-22(11-15)18(24)26-16-13-6-12-7-14(16)10-20(25,8-12)9-13/h12-16,25H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329303
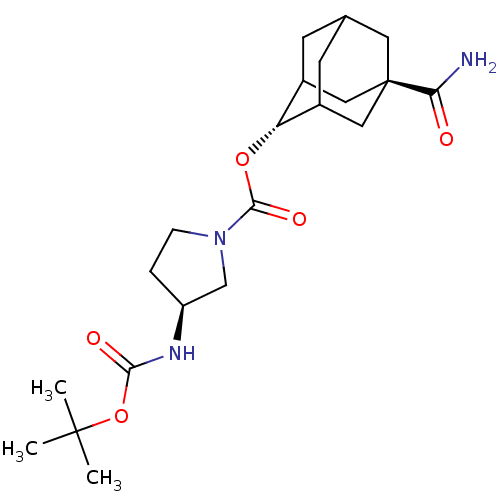
((S)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:16.16,wD:8.7,23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(7.71,5.47,;6.17,5.49,;5.42,6.84,;6.93,6.84,;5.38,4.17,;3.84,4.2,;3.09,5.54,;3.05,2.88,;1.51,2.9,;.62,4.16,;-.85,3.71,;-.88,2.16,;.58,1.67,;-2.22,1.41,;-2.23,-.13,;-3.55,2.19,;-4.89,1.43,;-4.9,-.1,;-5.91,-1.37,;-7.32,-.81,;-7.32,.77,;-6.28,2.01,;-7.63,1.53,;-7.62,.05,;-8.82,-1.23,;-6.29,-.44,;-8.96,.82,;-10.31,.05,;-8.96,2.36,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329311

((R)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.14,-33.31,;-8.79,-32.53,;-8.79,-30.99,;-7.45,-33.31,;-8.65,-34.59,;-7.15,-34.17,;-5.74,-34.73,;-4.73,-33.46,;-6.12,-33.8,;-4.72,-31.93,;-3.37,-31.17,;-2.05,-31.95,;-2.06,-33.49,;-.71,-31.19,;-.68,-29.65,;.79,-29.2,;1.68,-30.46,;.75,-31.69,;3.22,-30.48,;4.01,-29.16,;5.55,-29.19,;6.34,-27.87,;5.59,-26.53,;4.04,-26.51,;3.26,-27.83,;6.38,-25.2,;7.92,-25.23,;5.63,-23.86,;7.14,-23.86,;-6.11,-31.35,;-7.15,-32.58,;-7.46,-31.83,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329287
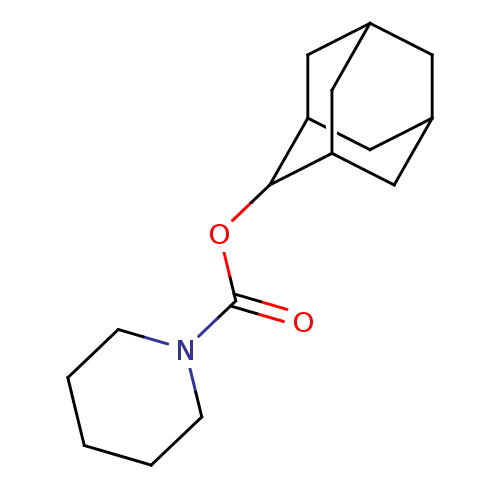
(CHEMBL1269124 | Piperidine-1-carboxylic acid adama...)Show SMILES O=C(OC1C2CC3CC(C2)CC1C3)N1CCCCC1 |TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(10.68,1.46,;10.69,3,;9.37,3.78,;8.02,3.03,;8.01,1.5,;6.62,1.15,;5.29,1.64,;4.09,.37,;5.59,.79,;7,.22,;5.59,2.37,;6.63,3.61,;5.28,3.13,;12.03,3.76,;13.36,2.97,;14.69,3.72,;14.71,5.26,;13.39,6.05,;12.04,5.3,)| Show InChI InChI=1S/C16H25NO2/c18-16(17-4-2-1-3-5-17)19-15-13-7-11-6-12(9-13)10-14(15)8-11/h11-15H,1-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329318

((S)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.94,-49.07,;25.29,-48.29,;25.29,-46.75,;26.62,-49.07,;25.43,-50.35,;26.93,-49.93,;28.33,-50.49,;29.35,-49.21,;27.95,-49.56,;29.36,-47.69,;30.7,-46.93,;32.03,-47.71,;32.01,-49.25,;33.37,-46.95,;33.39,-45.41,;34.87,-44.95,;35.75,-46.21,;34.83,-47.45,;37.29,-46.24,;38.08,-44.92,;39.62,-44.95,;40.41,-43.63,;39.67,-42.28,;38.12,-42.26,;37.33,-43.58,;40.45,-40.96,;41.24,-39.64,;27.96,-47.11,;26.92,-48.34,;26.62,-47.58,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329305
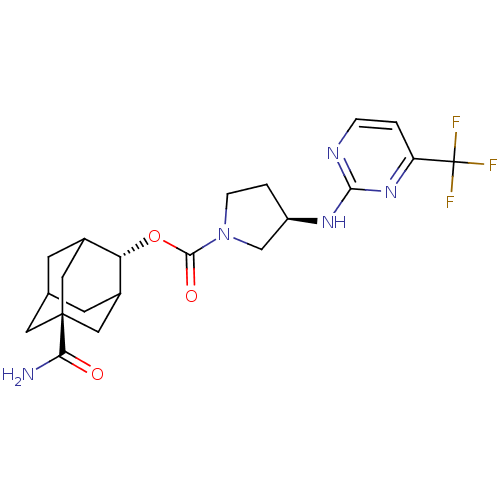
((R)-3-(4-Trifluoromethyl-pyrimidin-2-ylamino)-pyrr...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1nccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.48,-11.09,;-9.13,-10.31,;-9.13,-8.77,;-7.79,-11.09,;-8.99,-12.37,;-7.49,-11.95,;-6.08,-12.51,;-5.07,-11.23,;-6.46,-11.58,;-5.06,-9.71,;-3.72,-8.95,;-2.39,-9.73,;-2.41,-11.27,;-1.05,-8.97,;-1.02,-7.43,;.45,-6.98,;1.33,-8.24,;.41,-9.47,;2.87,-8.26,;3.67,-6.94,;5.21,-6.97,;6,-5.65,;5.25,-4.31,;3.7,-4.28,;2.92,-5.61,;2.95,-2.94,;3.73,-1.62,;1.41,-2.92,;2.16,-1.6,;-6.45,-9.13,;-7.49,-10.36,;-7.8,-9.61,)| Show InChI InChI=1S/C21H26F3N5O3/c22-21(23,24)15-1-3-26-18(28-15)27-14-2-4-29(10-14)19(31)32-16-12-5-11-6-13(16)9-20(7-11,8-12)17(25)30/h1,3,11-14,16H,2,4-10H2,(H2,25,30)(H,26,27,28)/t11?,12?,13?,14-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329291
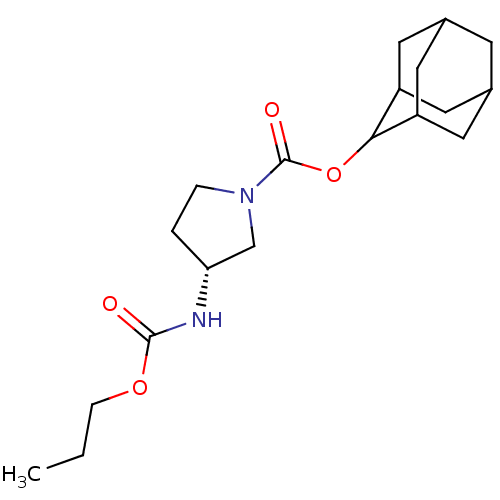
((R)-3-Propoxycarbonylamino-pyrrolidine-1-carboxyli...)Show SMILES CCCOC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:7.6,TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:15:16:19:22.23.24,15:23:19:17.21.16,14:15:19.20.22:24,(35.18,-1.92,;34.39,-3.24,;32.85,-3.22,;32.06,-4.54,;30.52,-4.51,;29.77,-3.17,;29.73,-5.83,;28.19,-5.81,;27.31,-4.55,;25.83,-5,;25.81,-6.55,;27.27,-7.04,;24.47,-7.3,;24.45,-8.84,;23.14,-6.52,;21.8,-7.28,;21.79,-8.81,;20.39,-9.15,;19.06,-8.66,;17.87,-9.94,;19.37,-9.52,;20.77,-10.09,;19.36,-7.94,;20.4,-6.7,;19.06,-7.18,)| Show InChI InChI=1S/C19H30N2O4/c1-2-5-24-18(22)20-16-3-4-21(11-16)19(23)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,2-11H2,1H3,(H,20,22)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329300
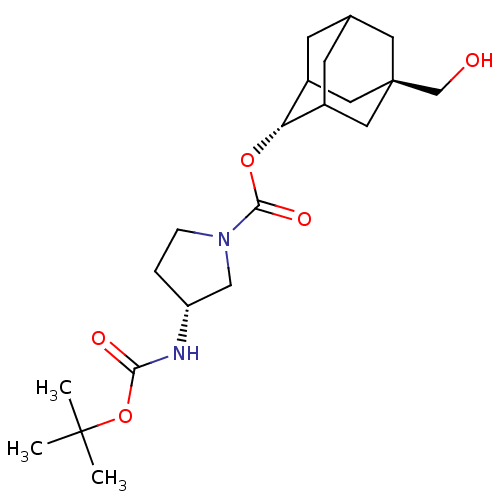
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](CO)(C3)C2 |r,wU:8.7,16.16,wD:23.25,TLB:16:17:26:20.21.22,15:16:26.19.20:22,THB:18:19:22:27.17.16,18:17:26.19.20:22,16:21:26:27.18.17,(8,-43.38,;6.46,-43.35,;5.71,-42,;7.22,-42.01,;5.66,-44.67,;4.12,-44.65,;3.38,-43.3,;3.33,-45.97,;1.79,-45.94,;.91,-44.68,;-.56,-45.13,;-.59,-46.68,;.87,-47.17,;-1.93,-47.44,;-1.95,-48.98,;-3.26,-46.66,;-4.6,-47.41,;-4.61,-48.94,;-5.62,-50.22,;-7.03,-49.65,;-7.03,-48.07,;-5.99,-46.83,;-7.34,-47.31,;-7.33,-48.8,;-8.67,-48.02,;-10.02,-48.79,;-8.53,-50.07,;-6.01,-49.29,)| Show InChI InChI=1S/C21H34N2O5/c1-20(2,3)28-18(25)22-16-4-5-23(11-16)19(26)27-17-14-6-13-7-15(17)10-21(8-13,9-14)12-24/h13-17,24H,4-12H2,1-3H3,(H,22,25)/t13?,14?,15?,16-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329290

((R)-3-Isopropoxycarbonylamino-pyrrolidine-1-carbox...)Show SMILES CC(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:7.6,TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:15:16:19:22.23.24,15:23:19:17.21.16,14:15:19.20.22:24,(16.75,-5.72,;15.21,-5.69,;14.46,-4.34,;14.41,-7.01,;12.87,-6.99,;12.13,-5.64,;12.08,-8.31,;10.54,-8.28,;9.66,-7.02,;8.19,-7.47,;8.16,-9.02,;9.62,-9.51,;6.82,-9.78,;6.8,-11.32,;5.49,-9,;4.15,-9.75,;4.14,-11.28,;2.74,-11.63,;1.42,-11.14,;.22,-12.41,;1.72,-11.99,;3.13,-12.56,;1.72,-10.41,;2.76,-9.17,;1.41,-9.65,)| Show InChI InChI=1S/C19H30N2O4/c1-11(2)24-18(22)20-16-3-4-21(10-16)19(23)25-17-14-6-12-5-13(8-14)9-15(17)7-12/h11-17H,3-10H2,1-2H3,(H,20,22)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329306

((R)-3-(2-Trifluoromethyl-pyrimidin-4-ylamino)-pyrr...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccnc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(6.77,-10.92,;8.12,-10.15,;8.12,-8.61,;9.46,-10.93,;8.26,-12.2,;9.76,-11.78,;11.17,-12.35,;12.18,-11.07,;10.79,-11.42,;12.19,-9.54,;13.53,-8.79,;14.86,-9.57,;14.85,-11.11,;16.2,-8.81,;16.23,-7.26,;17.7,-6.81,;18.59,-8.07,;17.66,-9.3,;20.13,-8.1,;20.92,-6.78,;22.46,-6.81,;23.25,-5.49,;22.5,-4.14,;20.95,-4.12,;20.17,-5.44,;20.2,-2.78,;20.99,-1.45,;18.66,-2.76,;19.41,-1.44,;10.8,-8.96,;9.76,-10.2,;9.45,-9.44,)| Show InChI InChI=1S/C21H26F3N5O3/c22-21(23,24)18-26-3-1-15(28-18)27-14-2-4-29(10-14)19(31)32-16-12-5-11-6-13(16)9-20(7-11,8-12)17(25)30/h1,3,11-14,16H,2,4-10H2,(H2,25,30)(H,26,27,28)/t11?,12?,13?,14-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329304
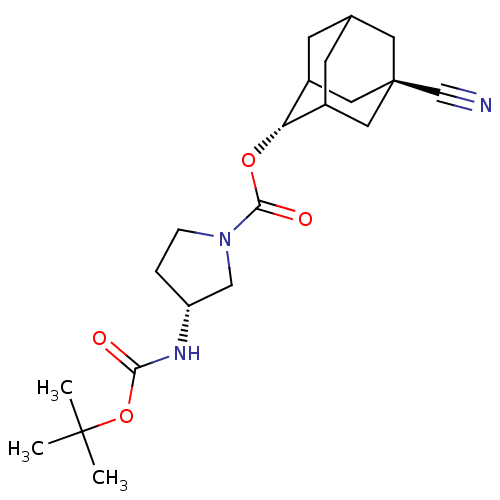
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C#N |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(26.4,5.69,;24.86,5.72,;24.11,7.07,;25.62,7.06,;24.07,4.4,;22.53,4.42,;21.78,5.77,;21.74,3.1,;20.2,3.13,;19.31,4.39,;17.84,3.94,;17.81,2.39,;19.27,1.9,;16.47,1.63,;16.46,.09,;15.14,2.41,;13.8,1.66,;13.79,.13,;12.78,-1.15,;11.37,-.58,;11.37,1,;12.41,2.24,;11.06,1.76,;11.07,.27,;9.87,-1,;12.4,-.22,;9.73,1.05,;8.39,1.83,)| Show InChI InChI=1S/C21H31N3O4/c1-20(2,3)28-18(25)23-16-4-5-24(11-16)19(26)27-17-14-6-13-7-15(17)10-21(8-13,9-14)12-22/h13-17H,4-11H2,1-3H3,(H,23,25)/t13?,14?,15?,16-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329317

((S)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(2.39,-49.17,;3.73,-48.39,;3.74,-46.85,;5.07,-49.17,;3.88,-50.45,;5.38,-50.03,;6.78,-50.59,;7.8,-49.31,;6.4,-49.66,;7.81,-47.79,;9.15,-47.03,;10.47,-47.81,;10.46,-49.35,;11.82,-47.05,;11.84,-45.51,;13.31,-45.06,;14.2,-46.32,;13.27,-47.55,;15.74,-46.34,;16.53,-45.02,;15.78,-43.69,;16.56,-42.36,;18.11,-42.39,;18.86,-43.73,;18.07,-45.05,;18.82,-46.4,;19.56,-47.74,;6.41,-47.21,;5.37,-48.44,;5.06,-47.69,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329293
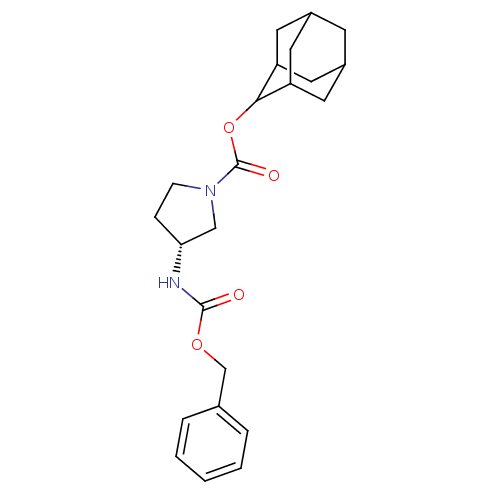
((R)-3-Benzyloxycarbonylamino-pyrrolidine-1-carboxy...)Show SMILES O=C(N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3)OCc1ccccc1 |r,wU:3.2,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:11:12:15:18.19.20,11:19:15:13.17.12,10:11:15.16.18:20,(15.71,-16.06,;16.45,-17.4,;15.66,-18.72,;14.12,-18.7,;13.24,-17.44,;11.77,-17.89,;11.74,-19.44,;13.2,-19.93,;10.4,-20.19,;10.38,-21.73,;9.07,-19.41,;7.73,-20.17,;7.72,-21.7,;6.32,-22.04,;4.99,-21.55,;3.8,-22.83,;5.3,-22.41,;6.7,-22.97,;5.29,-20.83,;6.33,-19.59,;4.99,-20.07,;17.99,-17.43,;18.79,-16.11,;20.33,-16.13,;21.07,-17.48,;22.61,-17.5,;23.4,-16.18,;22.64,-14.83,;21.11,-14.81,)| Show InChI InChI=1S/C23H30N2O4/c26-22(28-14-15-4-2-1-3-5-15)24-20-6-7-25(13-20)23(27)29-21-18-9-16-8-17(11-18)12-19(21)10-16/h1-5,16-21H,6-14H2,(H,24,26)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329289
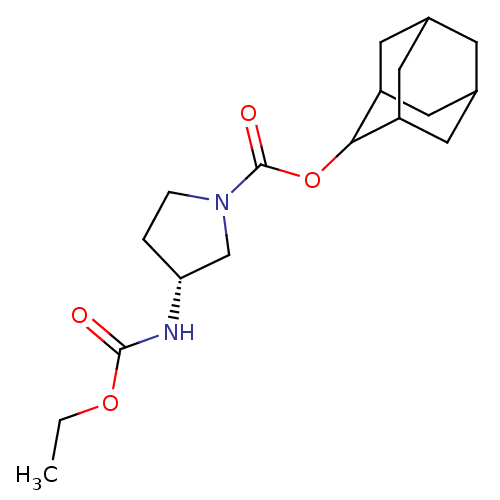
((R)-3-Ethoxycarbonylamino-pyrrolidine-1-carboxylic...)Show SMILES CCOC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:6.5,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(5.15,-3.67,;3.61,-3.64,;2.82,-4.97,;1.28,-4.94,;.53,-3.59,;.49,-6.26,;-1.05,-6.24,;-1.94,-4.98,;-3.41,-5.43,;-3.44,-6.97,;-1.98,-7.47,;-4.78,-7.73,;-4.79,-9.27,;-6.1,-6.95,;-7.45,-7.71,;-7.46,-9.24,;-8.85,-9.58,;-10.18,-9.09,;-11.38,-10.37,;-9.88,-9.95,;-8.47,-10.51,;-9.88,-8.36,;-8.84,-7.13,;-10.19,-7.61,)| Show InChI InChI=1S/C18H28N2O4/c1-2-23-17(21)19-15-3-4-20(10-15)18(22)24-16-13-6-11-5-12(8-13)9-14(16)7-11/h11-16H,2-10H2,1H3,(H,19,21)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329310

((R)-3-(4-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cc(ccn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(25.09,-21.61,;26.44,-20.84,;26.44,-19.3,;27.78,-21.61,;26.58,-22.89,;28.08,-22.47,;29.49,-23.04,;30.5,-21.76,;29.1,-22.1,;30.51,-20.23,;31.85,-19.47,;33.18,-20.25,;33.16,-21.79,;34.52,-19.5,;34.55,-17.95,;36.02,-17.5,;36.9,-18.76,;35.98,-19.99,;38.44,-18.78,;39.23,-17.46,;40.77,-17.5,;41.56,-16.18,;40.82,-14.83,;39.27,-14.81,;38.48,-16.13,;43.1,-16.2,;43.85,-17.55,;43.9,-14.88,;44.64,-16.2,;29.11,-19.65,;28.08,-20.89,;27.77,-20.13,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-3-27-17(7-15)28-16-2-4-29(11-16)20(31)32-18-13-5-12-6-14(18)10-21(8-12,9-13)19(26)30/h1,3,7,12-14,16,18H,2,4-6,8-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329288

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(33.72,4.21,;32.18,4.23,;31.44,5.58,;32.95,5.58,;31.39,2.91,;29.85,2.94,;29.11,4.28,;29.06,1.62,;27.52,1.64,;26.64,2.9,;25.17,2.45,;25.14,.9,;26.6,.41,;23.8,.15,;23.78,-1.39,;22.47,.93,;21.13,.17,;21.12,-1.36,;19.72,-1.7,;18.4,-1.21,;17.2,-2.49,;18.7,-2.07,;20.11,-2.63,;18.7,-.49,;19.73,.75,;18.39,.27,)| Show InChI InChI=1S/C20H32N2O4/c1-20(2,3)26-18(23)21-16-4-5-22(11-16)19(24)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329296
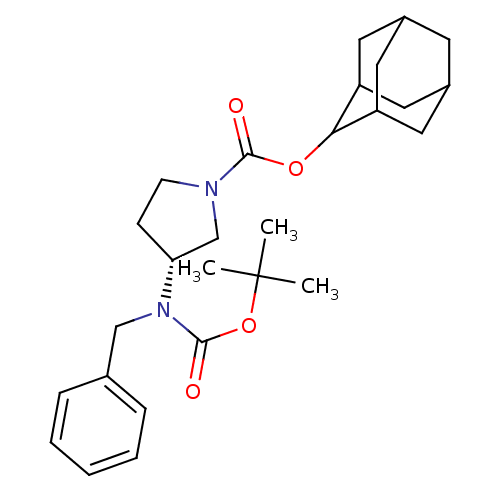
((R)-3-(Benzyl-tert-butoxycarbonyl-amino)-pyrrolidi...)Show SMILES CC(C)(C)OC(=O)N(Cc1ccccc1)[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:15.15,TLB:29:28:32:25.24.23,29:24:27.28.30:32,THB:23:24:27:30.31.32,23:31:27:25.29.24,22:23:27.28.30:32,(22.47,-24.3,;20.93,-24.28,;20.18,-22.93,;21.69,-22.94,;20.14,-25.6,;18.6,-25.58,;17.85,-24.23,;17.81,-26.9,;18.56,-28.24,;20.1,-28.27,;20.84,-29.61,;22.38,-29.64,;23.17,-28.32,;22.42,-26.97,;20.88,-26.95,;16.27,-26.87,;15.38,-25.61,;13.91,-26.06,;13.88,-27.61,;15.34,-28.1,;12.54,-28.37,;12.53,-29.91,;11.22,-27.58,;9.88,-28.34,;9.86,-29.87,;8.47,-30.22,;7.14,-29.73,;5.94,-31,;7.45,-30.58,;8.85,-31.15,;7.44,-29,;8.48,-27.76,;7.13,-28.24,)| Show InChI InChI=1S/C27H38N2O4/c1-27(2,3)33-26(31)29(16-18-7-5-4-6-8-18)23-9-10-28(17-23)25(30)32-24-21-12-19-11-20(14-21)15-22(24)13-19/h4-8,19-24H,9-17H2,1-3H3/t19?,20?,21?,22?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329297

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:8.7,16.16,wD:23.25,TLB:16:17:25:20.21.22,15:16:25.19.20:22,THB:18:19:22:26.17.16,18:17:25.19.20:22,16:21:25:26.18.17,(6.15,-33.79,;4.61,-33.76,;3.86,-32.41,;5.37,-32.42,;3.82,-35.08,;2.28,-35.06,;1.53,-33.71,;1.49,-36.38,;-.05,-36.35,;-.93,-35.09,;-2.41,-35.54,;-2.43,-37.09,;-.98,-37.58,;-3.78,-37.85,;-3.79,-39.39,;-5.1,-37.07,;-6.44,-37.82,;-6.45,-39.35,;-7.47,-40.63,;-8.87,-40.06,;-8.88,-38.48,;-7.84,-37.24,;-9.18,-37.72,;-9.18,-39.21,;-10.52,-38.43,;-10.37,-40.48,;-7.85,-39.7,)| Show InChI InChI=1S/C20H32N2O5/c1-19(2,3)27-17(23)21-15-4-5-22(11-15)18(24)26-16-13-6-12-7-14(16)10-20(25,8-12)9-13/h12-16,25H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329298
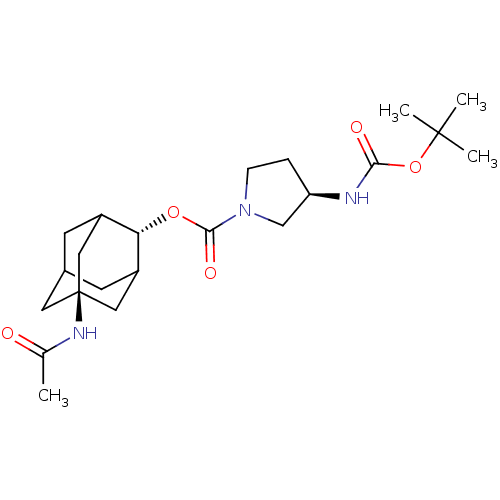
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(=O)N[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)NC(=O)OC(C)(C)C)C(C3)C2 |r,wU:17.20,10.11,wD:4.3,TLB:7:6:29:9.8.10,7:8:5.6.28:29,THB:10:8:5:28.27.29,10:27:5:9.7.8,11:10:5.6.28:29,(6.47,-37.02,;7.8,-37.8,;7.8,-39.34,;9.14,-37.03,;10.48,-37.81,;9.28,-39.08,;10.78,-38.66,;12.19,-39.23,;13.2,-37.95,;11.81,-38.3,;13.21,-36.42,;14.55,-35.67,;15.88,-36.45,;15.86,-37.99,;17.22,-35.69,;17.25,-34.14,;18.72,-33.69,;19.6,-34.95,;18.68,-36.18,;21.14,-34.98,;21.94,-33.66,;21.19,-32.31,;23.48,-33.68,;24.27,-32.36,;25.81,-32.39,;23.52,-31.01,;25.03,-31.02,;11.82,-35.84,;10.78,-37.08,;10.47,-36.32,)| Show InChI InChI=1S/C22H35N3O5/c1-13(26)24-22-9-14-7-15(10-22)18(16(8-14)11-22)29-20(28)25-6-5-17(12-25)23-19(27)30-21(2,3)4/h14-18H,5-12H2,1-4H3,(H,23,27)(H,24,26)/t14?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50306409
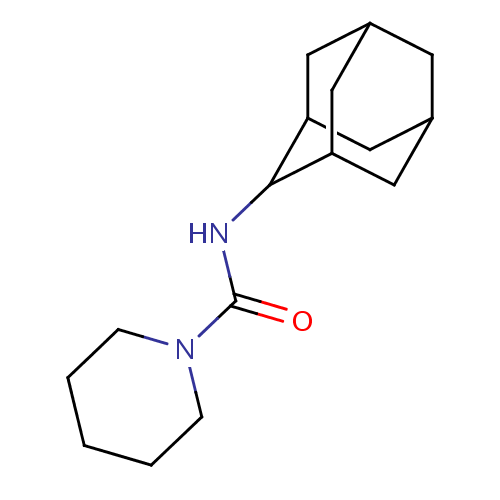
(CHEMBL596815 | Piperidine-1-carboxylic acid adaman...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)N1CCCCC1 |TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12| Show InChI InChI=1S/C16H26N2O/c19-16(18-4-2-1-3-5-18)17-15-13-7-11-6-12(9-13)10-14(15)8-11/h11-15H,1-10H2,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329305

((R)-3-(4-Trifluoromethyl-pyrimidin-2-ylamino)-pyrr...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1nccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.48,-11.09,;-9.13,-10.31,;-9.13,-8.77,;-7.79,-11.09,;-8.99,-12.37,;-7.49,-11.95,;-6.08,-12.51,;-5.07,-11.23,;-6.46,-11.58,;-5.06,-9.71,;-3.72,-8.95,;-2.39,-9.73,;-2.41,-11.27,;-1.05,-8.97,;-1.02,-7.43,;.45,-6.98,;1.33,-8.24,;.41,-9.47,;2.87,-8.26,;3.67,-6.94,;5.21,-6.97,;6,-5.65,;5.25,-4.31,;3.7,-4.28,;2.92,-5.61,;2.95,-2.94,;3.73,-1.62,;1.41,-2.92,;2.16,-1.6,;-6.45,-9.13,;-7.49,-10.36,;-7.8,-9.61,)| Show InChI InChI=1S/C21H26F3N5O3/c22-21(23,24)15-1-3-26-18(28-15)27-14-2-4-29(10-14)19(31)32-16-12-5-11-6-13(16)9-20(7-11,8-12)17(25)30/h1,3,11-14,16H,2,4-10H2,(H2,25,30)(H,26,27,28)/t11?,12?,13?,14-,16-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329303

((S)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:16.16,wD:8.7,23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(7.71,5.47,;6.17,5.49,;5.42,6.84,;6.93,6.84,;5.38,4.17,;3.84,4.2,;3.09,5.54,;3.05,2.88,;1.51,2.9,;.62,4.16,;-.85,3.71,;-.88,2.16,;.58,1.67,;-2.22,1.41,;-2.23,-.13,;-3.55,2.19,;-4.89,1.43,;-4.9,-.1,;-5.91,-1.37,;-7.32,-.81,;-7.32,.77,;-6.28,2.01,;-7.63,1.53,;-7.62,.05,;-8.82,-1.23,;-6.29,-.44,;-8.96,.82,;-10.31,.05,;-8.96,2.36,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329292

((R)-3-Isobutoxycarbonylamino-pyrrolidine-1-carboxy...)Show SMILES CC(C)COC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(5.77,-14.44,;4.98,-15.76,;5.73,-17.11,;3.44,-15.74,;2.65,-17.06,;1.11,-17.04,;.36,-15.69,;.32,-18.36,;-1.22,-18.33,;-2.11,-17.07,;-3.58,-17.52,;-3.61,-19.07,;-2.15,-19.56,;-4.95,-19.83,;-4.96,-21.37,;-6.28,-19.04,;-7.62,-19.8,;-7.63,-21.33,;-9.02,-21.68,;-10.35,-21.19,;-11.55,-22.46,;-10.05,-22.04,;-8.64,-22.61,;-10.05,-20.46,;-9.01,-19.22,;-10.36,-19.7,)| Show InChI InChI=1S/C20H32N2O4/c1-12(2)11-25-19(23)21-17-3-4-22(10-17)20(24)26-18-15-6-13-5-14(8-15)9-16(18)7-13/h12-18H,3-11H2,1-2H3,(H,21,23)/t13?,14?,15?,16?,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329307
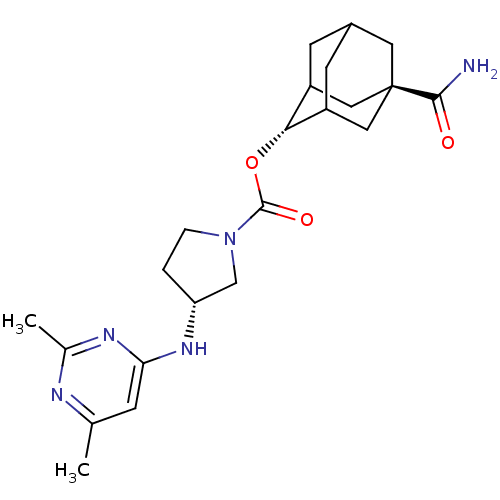
((R)-3-(2,6-Dimethyl-pyrimidin-4-ylamino)-pyrrolidi...)Show SMILES Cc1cc(N[C@@H]2CCN(C2)C(=O)O[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)nc(C)n1 |r,wU:5.4,13.13,wD:20.26,TLB:13:14:21:17.18.19,12:13:21.16.17:19,THB:15:16:19:22.14.13,15:14:21.16.17:19,13:18:21:22.15.14,(41.14,-5.48,;39.6,-5.45,;38.81,-6.77,;37.27,-6.74,;36.48,-8.06,;34.94,-8.03,;34.06,-6.77,;32.59,-7.23,;32.56,-8.77,;34.02,-9.26,;31.22,-9.53,;31.2,-11.07,;29.89,-8.75,;28.55,-9.5,;28.54,-11.03,;27.53,-12.31,;26.12,-11.74,;26.12,-10.16,;27.15,-8.92,;25.81,-9.4,;25.82,-10.89,;24.62,-12.16,;27.14,-11.38,;24.48,-10.11,;23.13,-10.88,;24.48,-8.57,;36.52,-5.4,;37.31,-4.08,;36.56,-2.74,;38.86,-4.1,)| Show InChI InChI=1S/C22H31N5O3/c1-12-5-18(25-13(2)24-12)26-17-3-4-27(11-17)21(29)30-19-15-6-14-7-16(19)10-22(8-14,9-15)20(23)28/h5,14-17,19H,3-4,6-11H2,1-2H3,(H2,23,28)(H,24,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329308
((R)-3-(5-Trifluoromethyl-[1,3,4]thiadiazol-2-ylami...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1nnc(s1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:30:8.7.9,6:7:4.5.29:30,THB:9:7:4:29.28.30,9:28:4:8.6.7,10:9:4.5.29:30,(-10.21,-23.19,;-8.86,-22.42,;-8.86,-20.88,;-7.52,-23.19,;-8.72,-24.47,;-7.22,-24.05,;-5.81,-24.61,;-4.8,-23.34,;-6.19,-23.68,;-4.79,-21.81,;-3.44,-21.05,;-2.12,-21.83,;-2.13,-23.37,;-.78,-21.08,;-.75,-19.53,;.72,-19.08,;1.61,-20.34,;.68,-21.57,;3.15,-20.36,;3.94,-19.04,;3.33,-17.62,;4.49,-16.61,;5.81,-17.4,;5.47,-18.9,;7.23,-16.8,;8.46,-17.72,;7.42,-15.27,;8.56,-16.01,;-6.18,-21.23,;-7.22,-22.47,;-7.53,-21.71,)| Show InChI InChI=1S/C19H24F3N5O3S/c20-19(21,22)15-25-26-16(31-15)24-12-1-2-27(8-12)17(29)30-13-10-3-9-4-11(13)7-18(5-9,6-10)14(23)28/h9-13H,1-8H2,(H2,23,28)(H,24,26)/t9?,10?,11?,12-,13-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329304

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C#N |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(26.4,5.69,;24.86,5.72,;24.11,7.07,;25.62,7.06,;24.07,4.4,;22.53,4.42,;21.78,5.77,;21.74,3.1,;20.2,3.13,;19.31,4.39,;17.84,3.94,;17.81,2.39,;19.27,1.9,;16.47,1.63,;16.46,.09,;15.14,2.41,;13.8,1.66,;13.79,.13,;12.78,-1.15,;11.37,-.58,;11.37,1,;12.41,2.24,;11.06,1.76,;11.07,.27,;9.87,-1,;12.4,-.22,;9.73,1.05,;8.39,1.83,)| Show InChI InChI=1S/C21H31N3O4/c1-20(2,3)28-18(25)23-16-4-5-24(11-16)19(26)27-17-14-6-13-7-15(17)10-21(8-13,9-14)12-22/h13-17H,4-11H2,1-3H3,(H,23,25)/t13?,14?,15?,16-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329295

((R)-3-(tert-Butoxycarbonyl-methyl-amino)-pyrrolidi...)Show SMILES CN([C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |r,wU:2.1,TLB:16:15:19:12.11.10,16:11:14.15.17:19,THB:10:11:14:17.18.19,10:18:14:12.16.11,9:10:14.15.17:19,(2.39,-29.14,;1.64,-27.79,;.1,-27.77,;-.79,-26.51,;-2.26,-26.96,;-2.29,-28.5,;-.83,-29,;-3.63,-29.26,;-3.64,-30.8,;-4.95,-28.48,;-6.29,-29.24,;-6.31,-30.76,;-7.7,-31.11,;-9.03,-30.62,;-10.23,-31.9,;-8.72,-31.48,;-7.32,-32.04,;-8.73,-29.89,;-7.69,-28.66,;-9.04,-29.14,;2.43,-26.47,;1.68,-25.12,;3.97,-26.49,;4.76,-25.17,;6.3,-25.2,;4.01,-23.83,;5.52,-23.83,)| Show InChI InChI=1S/C21H34N2O4/c1-21(2,3)27-19(24)22(4)17-5-6-23(12-17)20(25)26-18-15-8-13-7-14(10-15)11-16(18)9-13/h13-18H,5-12H2,1-4H3/t13?,14?,15?,16?,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329300

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](CO)(C3)C2 |r,wU:8.7,16.16,wD:23.25,TLB:16:17:26:20.21.22,15:16:26.19.20:22,THB:18:19:22:27.17.16,18:17:26.19.20:22,16:21:26:27.18.17,(8,-43.38,;6.46,-43.35,;5.71,-42,;7.22,-42.01,;5.66,-44.67,;4.12,-44.65,;3.38,-43.3,;3.33,-45.97,;1.79,-45.94,;.91,-44.68,;-.56,-45.13,;-.59,-46.68,;.87,-47.17,;-1.93,-47.44,;-1.95,-48.98,;-3.26,-46.66,;-4.6,-47.41,;-4.61,-48.94,;-5.62,-50.22,;-7.03,-49.65,;-7.03,-48.07,;-5.99,-46.83,;-7.34,-47.31,;-7.33,-48.8,;-8.67,-48.02,;-10.02,-48.79,;-8.53,-50.07,;-6.01,-49.29,)| Show InChI InChI=1S/C21H34N2O5/c1-20(2,3)28-18(25)22-16-4-5-23(11-16)19(26)27-17-14-6-13-7-15(17)10-21(8-13,9-14)12-24/h13-17,24H,4-12H2,1-3H3,(H,22,25)/t13?,14?,15?,16-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50306409

(CHEMBL596815 | Piperidine-1-carboxylic acid adaman...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)N1CCCCC1 |TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12| Show InChI InChI=1S/C16H26N2O/c19-16(18-4-2-1-3-5-18)17-15-13-7-11-6-12(9-13)10-14(15)8-11/h11-15H,1-10H2,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329290

((R)-3-Isopropoxycarbonylamino-pyrrolidine-1-carbox...)Show SMILES CC(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:7.6,TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:15:16:19:22.23.24,15:23:19:17.21.16,14:15:19.20.22:24,(16.75,-5.72,;15.21,-5.69,;14.46,-4.34,;14.41,-7.01,;12.87,-6.99,;12.13,-5.64,;12.08,-8.31,;10.54,-8.28,;9.66,-7.02,;8.19,-7.47,;8.16,-9.02,;9.62,-9.51,;6.82,-9.78,;6.8,-11.32,;5.49,-9,;4.15,-9.75,;4.14,-11.28,;2.74,-11.63,;1.42,-11.14,;.22,-12.41,;1.72,-11.99,;3.13,-12.56,;1.72,-10.41,;2.76,-9.17,;1.41,-9.65,)| Show InChI InChI=1S/C19H30N2O4/c1-11(2)24-18(22)20-16-3-4-21(10-16)19(23)25-17-14-6-12-5-13(8-14)9-15(17)7-12/h11-17H,3-10H2,1-2H3,(H,20,22)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329291

((R)-3-Propoxycarbonylamino-pyrrolidine-1-carboxyli...)Show SMILES CCCOC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:7.6,TLB:21:20:24:17.16.15,21:16:19.20.22:24,THB:15:16:19:22.23.24,15:23:19:17.21.16,14:15:19.20.22:24,(35.18,-1.92,;34.39,-3.24,;32.85,-3.22,;32.06,-4.54,;30.52,-4.51,;29.77,-3.17,;29.73,-5.83,;28.19,-5.81,;27.31,-4.55,;25.83,-5,;25.81,-6.55,;27.27,-7.04,;24.47,-7.3,;24.45,-8.84,;23.14,-6.52,;21.8,-7.28,;21.79,-8.81,;20.39,-9.15,;19.06,-8.66,;17.87,-9.94,;19.37,-9.52,;20.77,-10.09,;19.36,-7.94,;20.4,-6.7,;19.06,-7.18,)| Show InChI InChI=1S/C19H30N2O4/c1-2-5-24-18(22)20-16-3-4-21(11-16)19(23)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,2-11H2,1H3,(H,20,22)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329287

(CHEMBL1269124 | Piperidine-1-carboxylic acid adama...)Show SMILES O=C(OC1C2CC3CC(C2)CC1C3)N1CCCCC1 |TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(10.68,1.46,;10.69,3,;9.37,3.78,;8.02,3.03,;8.01,1.5,;6.62,1.15,;5.29,1.64,;4.09,.37,;5.59,.79,;7,.22,;5.59,2.37,;6.63,3.61,;5.28,3.13,;12.03,3.76,;13.36,2.97,;14.69,3.72,;14.71,5.26,;13.39,6.05,;12.04,5.3,)| Show InChI InChI=1S/C16H25NO2/c18-16(17-4-2-1-3-5-17)19-15-13-7-11-6-12(9-13)10-14(15)8-11/h11-15H,1-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329289

((R)-3-Ethoxycarbonylamino-pyrrolidine-1-carboxylic...)Show SMILES CCOC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:6.5,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:14:15:18:21.22.23,14:22:18:16.20.15,13:14:18.19.21:23,(5.15,-3.67,;3.61,-3.64,;2.82,-4.97,;1.28,-4.94,;.53,-3.59,;.49,-6.26,;-1.05,-6.24,;-1.94,-4.98,;-3.41,-5.43,;-3.44,-6.97,;-1.98,-7.47,;-4.78,-7.73,;-4.79,-9.27,;-6.1,-6.95,;-7.45,-7.71,;-7.46,-9.24,;-8.85,-9.58,;-10.18,-9.09,;-11.38,-10.37,;-9.88,-9.95,;-8.47,-10.51,;-9.88,-8.36,;-8.84,-7.13,;-10.19,-7.61,)| Show InChI InChI=1S/C18H28N2O4/c1-2-23-17(21)19-15-3-4-20(10-15)18(22)24-16-13-6-11-5-12(8-13)9-14(16)7-11/h11-16H,2-10H2,1H3,(H,19,21)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329298

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(=O)N[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)NC(=O)OC(C)(C)C)C(C3)C2 |r,wU:17.20,10.11,wD:4.3,TLB:7:6:29:9.8.10,7:8:5.6.28:29,THB:10:8:5:28.27.29,10:27:5:9.7.8,11:10:5.6.28:29,(6.47,-37.02,;7.8,-37.8,;7.8,-39.34,;9.14,-37.03,;10.48,-37.81,;9.28,-39.08,;10.78,-38.66,;12.19,-39.23,;13.2,-37.95,;11.81,-38.3,;13.21,-36.42,;14.55,-35.67,;15.88,-36.45,;15.86,-37.99,;17.22,-35.69,;17.25,-34.14,;18.72,-33.69,;19.6,-34.95,;18.68,-36.18,;21.14,-34.98,;21.94,-33.66,;21.19,-32.31,;23.48,-33.68,;24.27,-32.36,;25.81,-32.39,;23.52,-31.01,;25.03,-31.02,;11.82,-35.84,;10.78,-37.08,;10.47,-36.32,)| Show InChI InChI=1S/C22H35N3O5/c1-13(26)24-22-9-14-7-15(10-22)18(16(8-14)11-22)29-20(28)25-6-5-17(12-25)23-19(27)30-21(2,3)4/h14-18H,5-12H2,1-4H3,(H,23,27)(H,24,26)/t14?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329294
((R)-3-(3,3-Dimethyl-butyrylamino)-pyrrolidine-1-ca...)Show SMILES CC(C)(C)CC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(38.7,-14.79,;37.16,-14.77,;36.41,-13.42,;37.92,-13.42,;36.37,-16.09,;34.83,-16.06,;34.08,-14.72,;34.04,-17.38,;32.5,-17.36,;31.62,-16.1,;30.14,-16.55,;30.12,-18.1,;31.57,-18.59,;28.77,-18.85,;28.76,-20.39,;27.45,-18.07,;26.11,-18.83,;26.1,-20.36,;24.7,-20.7,;23.37,-20.21,;22.18,-21.49,;23.68,-21.07,;25.08,-21.64,;23.67,-19.49,;24.71,-18.25,;23.37,-18.73,)| Show InChI InChI=1S/C21H34N2O3/c1-21(2,3)11-18(24)22-17-4-5-23(12-17)20(25)26-19-15-7-13-6-14(9-15)10-16(19)8-13/h13-17,19H,4-12H2,1-3H3,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329293

((R)-3-Benzyloxycarbonylamino-pyrrolidine-1-carboxy...)Show SMILES O=C(N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3)OCc1ccccc1 |r,wU:3.2,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:11:12:15:18.19.20,11:19:15:13.17.12,10:11:15.16.18:20,(15.71,-16.06,;16.45,-17.4,;15.66,-18.72,;14.12,-18.7,;13.24,-17.44,;11.77,-17.89,;11.74,-19.44,;13.2,-19.93,;10.4,-20.19,;10.38,-21.73,;9.07,-19.41,;7.73,-20.17,;7.72,-21.7,;6.32,-22.04,;4.99,-21.55,;3.8,-22.83,;5.3,-22.41,;6.7,-22.97,;5.29,-20.83,;6.33,-19.59,;4.99,-20.07,;17.99,-17.43,;18.79,-16.11,;20.33,-16.13,;21.07,-17.48,;22.61,-17.5,;23.4,-16.18,;22.64,-14.83,;21.11,-14.81,)| Show InChI InChI=1S/C23H30N2O4/c26-22(28-14-15-4-2-1-3-5-15)24-20-6-7-25(13-20)23(27)29-21-18-9-16-8-17(11-18)12-19(21)10-16/h1-5,16-21H,6-14H2,(H,24,26)/t16?,17?,18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 84.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329301
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(C)(C)O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(22.08,-44.56,;20.54,-44.53,;19.79,-43.19,;21.3,-43.19,;19.75,-45.85,;18.21,-45.83,;17.46,-44.48,;17.42,-47.15,;15.88,-47.12,;14.99,-45.86,;13.52,-46.32,;13.5,-47.86,;14.95,-48.36,;12.15,-48.62,;12.14,-50.16,;10.83,-47.84,;9.49,-48.6,;9.48,-50.12,;8.46,-51.4,;7.06,-50.84,;7.05,-49.25,;8.09,-48.02,;6.74,-48.49,;6.75,-49.98,;5.56,-51.26,;8.08,-50.47,;5.41,-49.2,;4.64,-47.86,;6.18,-47.86,;4.07,-49.98,)| Show InChI InChI=1S/C23H38N2O5/c1-21(2,3)30-19(26)24-17-6-7-25(13-17)20(27)29-18-15-8-14-9-16(18)12-23(10-14,11-15)22(4,5)28/h14-18,28H,6-13H2,1-5H3,(H,24,26)/t14?,15?,16?,17-,18-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329299
((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES COC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)NC(=O)OC(C)(C)C)C(C3)C2 |r,wU:17.20,10.11,wD:4.3,TLB:7:6:29:9.8.10,7:8:5.6.28:29,THB:10:8:5:28.27.29,10:27:5:9.7.8,11:10:5.6.28:29,(22.87,-36.96,;24.2,-37.73,;25.55,-36.96,;25.55,-35.41,;26.89,-37.74,;25.69,-39.01,;27.19,-38.59,;28.6,-39.16,;29.61,-37.88,;28.22,-38.23,;29.62,-36.35,;30.96,-35.6,;32.29,-36.38,;32.28,-37.92,;33.63,-35.62,;33.66,-34.07,;35.13,-33.62,;36.02,-34.88,;35.09,-36.11,;37.56,-34.91,;38.35,-33.59,;37.6,-32.24,;39.89,-33.61,;40.68,-32.29,;42.22,-32.32,;39.93,-30.94,;41.44,-30.95,;28.23,-35.77,;27.19,-37.01,;26.88,-36.25,)| Show InChI InChI=1S/C22H34N2O6/c1-21(2,3)30-19(26)23-16-5-6-24(12-16)20(27)29-17-14-7-13-8-15(17)11-22(9-13,10-14)18(25)28-4/h13-17H,5-12H2,1-4H3,(H,23,26)/t13?,14?,15?,16-,17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329301

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(C)(C)O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(22.08,-44.56,;20.54,-44.53,;19.79,-43.19,;21.3,-43.19,;19.75,-45.85,;18.21,-45.83,;17.46,-44.48,;17.42,-47.15,;15.88,-47.12,;14.99,-45.86,;13.52,-46.32,;13.5,-47.86,;14.95,-48.36,;12.15,-48.62,;12.14,-50.16,;10.83,-47.84,;9.49,-48.6,;9.48,-50.12,;8.46,-51.4,;7.06,-50.84,;7.05,-49.25,;8.09,-48.02,;6.74,-48.49,;6.75,-49.98,;5.56,-51.26,;8.08,-50.47,;5.41,-49.2,;4.64,-47.86,;6.18,-47.86,;4.07,-49.98,)| Show InChI InChI=1S/C23H38N2O5/c1-21(2,3)30-19(26)24-17-6-7-25(13-17)20(27)29-18-15-8-14-9-16(18)12-23(10-14,11-15)22(4,5)28/h14-18,28H,6-13H2,1-5H3,(H,24,26)/t14?,15?,16?,17-,18-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329308

((R)-3-(5-Trifluoromethyl-[1,3,4]thiadiazol-2-ylami...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1nnc(s1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:30:8.7.9,6:7:4.5.29:30,THB:9:7:4:29.28.30,9:28:4:8.6.7,10:9:4.5.29:30,(-10.21,-23.19,;-8.86,-22.42,;-8.86,-20.88,;-7.52,-23.19,;-8.72,-24.47,;-7.22,-24.05,;-5.81,-24.61,;-4.8,-23.34,;-6.19,-23.68,;-4.79,-21.81,;-3.44,-21.05,;-2.12,-21.83,;-2.13,-23.37,;-.78,-21.08,;-.75,-19.53,;.72,-19.08,;1.61,-20.34,;.68,-21.57,;3.15,-20.36,;3.94,-19.04,;3.33,-17.62,;4.49,-16.61,;5.81,-17.4,;5.47,-18.9,;7.23,-16.8,;8.46,-17.72,;7.42,-15.27,;8.56,-16.01,;-6.18,-21.23,;-7.22,-22.47,;-7.53,-21.71,)| Show InChI InChI=1S/C19H24F3N5O3S/c20-19(21,22)15-25-26-16(31-15)24-12-1-2-27(8-12)17(29)30-13-10-3-9-4-11(13)7-18(5-9,6-10)14(23)28/h9-13H,1-8H2,(H2,23,28)(H,24,26)/t9?,10?,11?,12-,13-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329307

((R)-3-(2,6-Dimethyl-pyrimidin-4-ylamino)-pyrrolidi...)Show SMILES Cc1cc(N[C@@H]2CCN(C2)C(=O)O[C@H]2C3CC4CC2C[C@](C4)(C3)C(N)=O)nc(C)n1 |r,wU:5.4,13.13,wD:20.26,TLB:13:14:21:17.18.19,12:13:21.16.17:19,THB:15:16:19:22.14.13,15:14:21.16.17:19,13:18:21:22.15.14,(41.14,-5.48,;39.6,-5.45,;38.81,-6.77,;37.27,-6.74,;36.48,-8.06,;34.94,-8.03,;34.06,-6.77,;32.59,-7.23,;32.56,-8.77,;34.02,-9.26,;31.22,-9.53,;31.2,-11.07,;29.89,-8.75,;28.55,-9.5,;28.54,-11.03,;27.53,-12.31,;26.12,-11.74,;26.12,-10.16,;27.15,-8.92,;25.81,-9.4,;25.82,-10.89,;24.62,-12.16,;27.14,-11.38,;24.48,-10.11,;23.13,-10.88,;24.48,-8.57,;36.52,-5.4,;37.31,-4.08,;36.56,-2.74,;38.86,-4.1,)| Show InChI InChI=1S/C22H31N5O3/c1-12-5-18(25-13(2)24-12)26-17-3-4-27(11-17)21(29)30-19-15-6-14-7-16(19)10-22(8-14,9-15)20(23)28/h5,14-17,19H,3-4,6-11H2,1-2H3,(H2,23,28)(H,24,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329299

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES COC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)NC(=O)OC(C)(C)C)C(C3)C2 |r,wU:17.20,10.11,wD:4.3,TLB:7:6:29:9.8.10,7:8:5.6.28:29,THB:10:8:5:28.27.29,10:27:5:9.7.8,11:10:5.6.28:29,(22.87,-36.96,;24.2,-37.73,;25.55,-36.96,;25.55,-35.41,;26.89,-37.74,;25.69,-39.01,;27.19,-38.59,;28.6,-39.16,;29.61,-37.88,;28.22,-38.23,;29.62,-36.35,;30.96,-35.6,;32.29,-36.38,;32.28,-37.92,;33.63,-35.62,;33.66,-34.07,;35.13,-33.62,;36.02,-34.88,;35.09,-36.11,;37.56,-34.91,;38.35,-33.59,;37.6,-32.24,;39.89,-33.61,;40.68,-32.29,;42.22,-32.32,;39.93,-30.94,;41.44,-30.95,;28.23,-35.77,;27.19,-37.01,;26.88,-36.25,)| Show InChI InChI=1S/C22H34N2O6/c1-21(2,3)30-19(26)23-16-5-6-24(12-16)20(27)29-17-14-7-13-8-15(17)11-22(9-13,10-14)18(25)28-4/h13-17H,5-12H2,1-4H3,(H,23,26)/t13?,14?,15?,16-,17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 11betaHSD1 expressed in CHO cells assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50329296

((R)-3-(Benzyl-tert-butoxycarbonyl-amino)-pyrrolidi...)Show SMILES CC(C)(C)OC(=O)N(Cc1ccccc1)[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:15.15,TLB:29:28:32:25.24.23,29:24:27.28.30:32,THB:23:24:27:30.31.32,23:31:27:25.29.24,22:23:27.28.30:32,(22.47,-24.3,;20.93,-24.28,;20.18,-22.93,;21.69,-22.94,;20.14,-25.6,;18.6,-25.58,;17.85,-24.23,;17.81,-26.9,;18.56,-28.24,;20.1,-28.27,;20.84,-29.61,;22.38,-29.64,;23.17,-28.32,;22.42,-26.97,;20.88,-26.95,;16.27,-26.87,;15.38,-25.61,;13.91,-26.06,;13.88,-27.61,;15.34,-28.1,;12.54,-28.37,;12.53,-29.91,;11.22,-27.58,;9.88,-28.34,;9.86,-29.87,;8.47,-30.22,;7.14,-29.73,;5.94,-31,;7.45,-30.58,;8.85,-31.15,;7.44,-29,;8.48,-27.76,;7.13,-28.24,)| Show InChI InChI=1S/C27H38N2O4/c1-27(2,3)33-26(31)29(16-18-7-5-4-6-8-18)23-9-10-28(17-23)25(30)32-24-21-12-19-11-20(14-21)15-22(24)13-19/h4-8,19-24H,9-17H2,1-3H3/t19?,20?,21?,22?,23-,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human platelet assessed as [3H]-cortisone to [3H]-cortisol by microscintillation plate reader |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329314

((S)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-11.32,-46.08,;-9.96,-45.31,;-9.95,-43.77,;-8.62,-46.08,;-9.81,-47.36,;-8.31,-46.94,;-6.91,-47.5,;-5.89,-46.23,;-7.29,-46.57,;-5.88,-44.7,;-4.54,-43.94,;-3.22,-44.72,;-3.23,-46.26,;-1.87,-43.97,;-1.85,-42.42,;-.37,-41.97,;.51,-43.23,;-.42,-44.46,;2.05,-43.25,;2.84,-41.93,;4.38,-41.96,;5.17,-40.64,;4.42,-39.3,;2.88,-39.28,;2.09,-40.6,;5.21,-37.98,;6.75,-38,;4.46,-36.63,;5.97,-36.63,;-7.28,-44.12,;-8.32,-45.36,;-8.62,-44.6,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329312

((R)-3-(6-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(4.49,-33.95,;5.83,-33.18,;5.84,-31.64,;7.17,-33.96,;5.98,-35.23,;7.48,-34.81,;8.88,-35.38,;9.9,-34.1,;8.5,-34.45,;9.91,-32.57,;11.25,-31.82,;12.57,-32.6,;12.56,-34.14,;13.92,-31.84,;13.94,-30.29,;15.41,-29.84,;16.3,-31.1,;15.37,-32.33,;17.84,-31.13,;18.63,-29.81,;20.17,-29.84,;20.96,-28.52,;20.21,-27.17,;18.66,-27.15,;17.88,-28.47,;17.91,-25.81,;18.7,-24.48,;16.37,-25.79,;17.13,-24.47,;8.51,-31.99,;7.47,-33.23,;7.16,-32.47,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-3-17(28-16)27-15-4-5-29(11-15)20(31)32-18-13-6-12-7-14(18)10-21(8-12,9-13)19(26)30/h1-3,12-15,18H,4-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329309

((R)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(7.67,-22.53,;9.01,-21.75,;9.02,-20.21,;10.35,-22.53,;9.16,-23.81,;10.66,-23.39,;12.06,-23.95,;13.08,-22.68,;11.68,-23.02,;13.09,-21.15,;14.43,-20.39,;15.75,-21.17,;15.74,-22.71,;17.1,-20.41,;17.12,-18.87,;18.6,-18.42,;19.48,-19.68,;18.55,-20.91,;21.02,-19.7,;21.81,-18.38,;21.06,-17.05,;21.85,-15.73,;23.39,-15.75,;24.14,-17.09,;23.35,-18.41,;24.1,-19.76,;23.3,-21.08,;25.64,-19.79,;24.86,-21.09,;11.69,-20.57,;10.65,-21.8,;10.35,-21.05,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329311

((R)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.14,-33.31,;-8.79,-32.53,;-8.79,-30.99,;-7.45,-33.31,;-8.65,-34.59,;-7.15,-34.17,;-5.74,-34.73,;-4.73,-33.46,;-6.12,-33.8,;-4.72,-31.93,;-3.37,-31.17,;-2.05,-31.95,;-2.06,-33.49,;-.71,-31.19,;-.68,-29.65,;.79,-29.2,;1.68,-30.46,;.75,-31.69,;3.22,-30.48,;4.01,-29.16,;5.55,-29.19,;6.34,-27.87,;5.59,-26.53,;4.04,-26.51,;3.26,-27.83,;6.38,-25.2,;7.92,-25.23,;5.63,-23.86,;7.14,-23.86,;-6.11,-31.35,;-7.15,-32.58,;-7.46,-31.83,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329312

((R)-3-(6-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(4.49,-33.95,;5.83,-33.18,;5.84,-31.64,;7.17,-33.96,;5.98,-35.23,;7.48,-34.81,;8.88,-35.38,;9.9,-34.1,;8.5,-34.45,;9.91,-32.57,;11.25,-31.82,;12.57,-32.6,;12.56,-34.14,;13.92,-31.84,;13.94,-30.29,;15.41,-29.84,;16.3,-31.1,;15.37,-32.33,;17.84,-31.13,;18.63,-29.81,;20.17,-29.84,;20.96,-28.52,;20.21,-27.17,;18.66,-27.15,;17.88,-28.47,;17.91,-25.81,;18.7,-24.48,;16.37,-25.79,;17.13,-24.47,;8.51,-31.99,;7.47,-33.23,;7.16,-32.47,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-3-17(28-16)27-15-4-5-29(11-15)20(31)32-18-13-6-12-7-14(18)10-21(8-12,9-13)19(26)30/h1-3,12-15,18H,4-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329312

((R)-3-(6-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1cccc(n1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(4.49,-33.95,;5.83,-33.18,;5.84,-31.64,;7.17,-33.96,;5.98,-35.23,;7.48,-34.81,;8.88,-35.38,;9.9,-34.1,;8.5,-34.45,;9.91,-32.57,;11.25,-31.82,;12.57,-32.6,;12.56,-34.14,;13.92,-31.84,;13.94,-30.29,;15.41,-29.84,;16.3,-31.1,;15.37,-32.33,;17.84,-31.13,;18.63,-29.81,;20.17,-29.84,;20.96,-28.52,;20.21,-27.17,;18.66,-27.15,;17.88,-28.47,;17.91,-25.81,;18.7,-24.48,;16.37,-25.79,;17.13,-24.47,;8.51,-31.99,;7.47,-33.23,;7.16,-32.47,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-3-17(28-16)27-15-4-5-29(11-15)20(31)32-18-13-6-12-7-14(18)10-21(8-12,9-13)19(26)30/h1-3,12-15,18H,4-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,18-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329311

((R)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.14,-33.31,;-8.79,-32.53,;-8.79,-30.99,;-7.45,-33.31,;-8.65,-34.59,;-7.15,-34.17,;-5.74,-34.73,;-4.73,-33.46,;-6.12,-33.8,;-4.72,-31.93,;-3.37,-31.17,;-2.05,-31.95,;-2.06,-33.49,;-.71,-31.19,;-.68,-29.65,;.79,-29.2,;1.68,-30.46,;.75,-31.69,;3.22,-30.48,;4.01,-29.16,;5.55,-29.19,;6.34,-27.87,;5.59,-26.53,;4.04,-26.51,;3.26,-27.83,;6.38,-25.2,;7.92,-25.23,;5.63,-23.86,;7.14,-23.86,;-6.11,-31.35,;-7.15,-32.58,;-7.46,-31.83,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329313

((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wD:16.19,3.2,9.10,TLB:30:29:4.5.6:8,THB:30:5:8:31.29.9,9:29:4:6.7.8,9:7:4:31.30.29,10:9:4.5.6:8,(23.92,-31.27,;25.27,-30.5,;25.27,-28.96,;26.61,-31.27,;25.41,-32.55,;26.91,-32.13,;26.91,-30.55,;27.95,-29.31,;26.6,-29.79,;29.34,-29.89,;30.68,-29.13,;32.01,-29.91,;32,-31.45,;33.35,-29.16,;33.38,-27.61,;34.85,-27.16,;35.74,-28.42,;34.81,-29.65,;37.28,-28.44,;38.07,-27.12,;37.32,-25.79,;38.1,-24.47,;39.65,-24.49,;40.4,-25.84,;39.61,-27.16,;40.35,-28.5,;39.56,-29.82,;41.89,-28.53,;41.11,-29.83,;29.33,-31.42,;28.32,-32.7,;27.94,-31.76,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329313

((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wD:16.19,3.2,9.10,TLB:30:29:4.5.6:8,THB:30:5:8:31.29.9,9:29:4:6.7.8,9:7:4:31.30.29,10:9:4.5.6:8,(23.92,-31.27,;25.27,-30.5,;25.27,-28.96,;26.61,-31.27,;25.41,-32.55,;26.91,-32.13,;26.91,-30.55,;27.95,-29.31,;26.6,-29.79,;29.34,-29.89,;30.68,-29.13,;32.01,-29.91,;32,-31.45,;33.35,-29.16,;33.38,-27.61,;34.85,-27.16,;35.74,-28.42,;34.81,-29.65,;37.28,-28.44,;38.07,-27.12,;37.32,-25.79,;38.1,-24.47,;39.65,-24.49,;40.4,-25.84,;39.61,-27.16,;40.35,-28.5,;39.56,-29.82,;41.89,-28.53,;41.11,-29.83,;29.33,-31.42,;28.32,-32.7,;27.94,-31.76,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329309

((R)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(7.67,-22.53,;9.01,-21.75,;9.02,-20.21,;10.35,-22.53,;9.16,-23.81,;10.66,-23.39,;12.06,-23.95,;13.08,-22.68,;11.68,-23.02,;13.09,-21.15,;14.43,-20.39,;15.75,-21.17,;15.74,-22.71,;17.1,-20.41,;17.12,-18.87,;18.6,-18.42,;19.48,-19.68,;18.55,-20.91,;21.02,-19.7,;21.81,-18.38,;21.06,-17.05,;21.85,-15.73,;23.39,-15.75,;24.14,-17.09,;23.35,-18.41,;24.1,-19.76,;23.3,-21.08,;25.64,-19.79,;24.86,-21.09,;11.69,-20.57,;10.65,-21.8,;10.35,-21.05,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 2
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 3betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 17betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 17betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
3 beta-hydroxysteroid dehydrogenase/Delta 5-->4-isomerase type 2
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 3betaHSD2 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329314

((S)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-11.32,-46.08,;-9.96,-45.31,;-9.95,-43.77,;-8.62,-46.08,;-9.81,-47.36,;-8.31,-46.94,;-6.91,-47.5,;-5.89,-46.23,;-7.29,-46.57,;-5.88,-44.7,;-4.54,-43.94,;-3.22,-44.72,;-3.23,-46.26,;-1.87,-43.97,;-1.85,-42.42,;-.37,-41.97,;.51,-43.23,;-.42,-44.46,;2.05,-43.25,;2.84,-41.93,;4.38,-41.96,;5.17,-40.64,;4.42,-39.3,;2.88,-39.28,;2.09,-40.6,;5.21,-37.98,;6.75,-38,;4.46,-36.63,;5.97,-36.63,;-7.28,-44.12,;-8.32,-45.36,;-8.62,-44.6,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329288

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(33.72,4.21,;32.18,4.23,;31.44,5.58,;32.95,5.58,;31.39,2.91,;29.85,2.94,;29.11,4.28,;29.06,1.62,;27.52,1.64,;26.64,2.9,;25.17,2.45,;25.14,.9,;26.6,.41,;23.8,.15,;23.78,-1.39,;22.47,.93,;21.13,.17,;21.12,-1.36,;19.72,-1.7,;18.4,-1.21,;17.2,-2.49,;18.7,-2.07,;20.11,-2.63,;18.7,-.49,;19.73,.75,;18.39,.27,)| Show InChI InChI=1S/C20H32N2O4/c1-20(2,3)26-18(23)21-16-4-5-22(11-16)19(24)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329314

((S)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-11.32,-46.08,;-9.96,-45.31,;-9.95,-43.77,;-8.62,-46.08,;-9.81,-47.36,;-8.31,-46.94,;-6.91,-47.5,;-5.89,-46.23,;-7.29,-46.57,;-5.88,-44.7,;-4.54,-43.94,;-3.22,-44.72,;-3.23,-46.26,;-1.87,-43.97,;-1.85,-42.42,;-.37,-41.97,;.51,-43.23,;-.42,-44.46,;2.05,-43.25,;2.84,-41.93,;4.38,-41.96,;5.17,-40.64,;4.42,-39.3,;2.88,-39.28,;2.09,-40.6,;5.21,-37.98,;6.75,-38,;4.46,-36.63,;5.97,-36.63,;-7.28,-44.12,;-8.32,-45.36,;-8.62,-44.6,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329311

((R)-3-(5-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C(F)(F)F)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:31:8.7.9,6:7:4.5.30:31,THB:9:7:4:30.29.31,9:29:4:8.6.7,10:9:4.5.30:31,(-10.14,-33.31,;-8.79,-32.53,;-8.79,-30.99,;-7.45,-33.31,;-8.65,-34.59,;-7.15,-34.17,;-5.74,-34.73,;-4.73,-33.46,;-6.12,-33.8,;-4.72,-31.93,;-3.37,-31.17,;-2.05,-31.95,;-2.06,-33.49,;-.71,-31.19,;-.68,-29.65,;.79,-29.2,;1.68,-30.46,;.75,-31.69,;3.22,-30.48,;4.01,-29.16,;5.55,-29.19,;6.34,-27.87,;5.59,-26.53,;4.04,-26.51,;3.26,-27.83,;6.38,-25.2,;7.92,-25.23,;5.63,-23.86,;7.14,-23.86,;-6.11,-31.35,;-7.15,-32.58,;-7.46,-31.83,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)15-1-2-17(27-10-15)28-16-3-4-29(11-16)20(31)32-18-13-5-12-6-14(18)9-21(7-12,8-13)19(26)30/h1-2,10,12-14,16,18H,3-9,11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,16-,18-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329318

((S)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.94,-49.07,;25.29,-48.29,;25.29,-46.75,;26.62,-49.07,;25.43,-50.35,;26.93,-49.93,;28.33,-50.49,;29.35,-49.21,;27.95,-49.56,;29.36,-47.69,;30.7,-46.93,;32.03,-47.71,;32.01,-49.25,;33.37,-46.95,;33.39,-45.41,;34.87,-44.95,;35.75,-46.21,;34.83,-47.45,;37.29,-46.24,;38.08,-44.92,;39.62,-44.95,;40.41,-43.63,;39.67,-42.28,;38.12,-42.26,;37.33,-43.58,;40.45,-40.96,;41.24,-39.64,;27.96,-47.11,;26.92,-48.34,;26.62,-47.58,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329317

((S)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(2.39,-49.17,;3.73,-48.39,;3.74,-46.85,;5.07,-49.17,;3.88,-50.45,;5.38,-50.03,;6.78,-50.59,;7.8,-49.31,;6.4,-49.66,;7.81,-47.79,;9.15,-47.03,;10.47,-47.81,;10.46,-49.35,;11.82,-47.05,;11.84,-45.51,;13.31,-45.06,;14.2,-46.32,;13.27,-47.55,;15.74,-46.34,;16.53,-45.02,;15.78,-43.69,;16.56,-42.36,;18.11,-42.39,;18.86,-43.73,;18.07,-45.05,;18.82,-46.4,;19.56,-47.74,;6.41,-47.21,;5.37,-48.44,;5.06,-47.69,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329318

((S)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.94,-49.07,;25.29,-48.29,;25.29,-46.75,;26.62,-49.07,;25.43,-50.35,;26.93,-49.93,;28.33,-50.49,;29.35,-49.21,;27.95,-49.56,;29.36,-47.69,;30.7,-46.93,;32.03,-47.71,;32.01,-49.25,;33.37,-46.95,;33.39,-45.41,;34.87,-44.95,;35.75,-46.21,;34.83,-47.45,;37.29,-46.24,;38.08,-44.92,;39.62,-44.95,;40.41,-43.63,;39.67,-42.28,;38.12,-42.26,;37.33,-43.58,;40.45,-40.96,;41.24,-39.64,;27.96,-47.11,;26.92,-48.34,;26.62,-47.58,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329319
(Adamantan-2-ol | CHEMBL1269176)Show SMILES OC1C2CC3CC(C2)CC1C3 |TLB:7:6:10:3.2.1,7:2:5.6.8:10,THB:1:2:5:8.9.10,1:9:5:3.7.2,0:1:5.6.8:10,(32.02,1.86,;30.67,1.1,;30.65,-.44,;29.25,-.79,;27.91,-.3,;26.7,-1.58,;28.22,-1.16,;29.63,-1.73,;28.21,.44,;29.26,1.68,;27.9,1.2,)| Show InChI InChI=1S/C10H16O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-11H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329316

((R)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.78,-45.32,;25.12,-44.54,;25.13,-43,;26.46,-45.32,;25.26,-46.6,;26.77,-46.18,;28.17,-46.74,;29.18,-45.46,;27.79,-45.81,;29.2,-43.94,;30.54,-43.18,;31.86,-43.96,;31.85,-45.5,;33.2,-43.2,;33.23,-41.66,;34.7,-41.21,;35.59,-42.47,;34.66,-43.7,;37.13,-42.49,;37.92,-41.17,;39.46,-41.2,;40.25,-39.88,;39.5,-38.54,;37.95,-38.51,;37.17,-39.84,;40.29,-37.21,;41.08,-35.89,;27.8,-43.36,;26.76,-44.59,;26.45,-43.84,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329318

((S)-3-(5-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ccc(cn1)C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(23.94,-49.07,;25.29,-48.29,;25.29,-46.75,;26.62,-49.07,;25.43,-50.35,;26.93,-49.93,;28.33,-50.49,;29.35,-49.21,;27.95,-49.56,;29.36,-47.69,;30.7,-46.93,;32.03,-47.71,;32.01,-49.25,;33.37,-46.95,;33.39,-45.41,;34.87,-44.95,;35.75,-46.21,;34.83,-47.45,;37.29,-46.24,;38.08,-44.92,;39.62,-44.95,;40.41,-43.63,;39.67,-42.28,;38.12,-42.26,;37.33,-43.58,;40.45,-40.96,;41.24,-39.64,;27.96,-47.11,;26.92,-48.34,;26.62,-47.58,)| Show InChI InChI=1S/C22H27N5O3/c23-10-13-1-2-18(25-11-13)26-17-3-4-27(12-17)21(29)30-19-15-5-14-6-16(19)9-22(7-14,8-15)20(24)28/h1-2,11,14-17,19H,3-9,12H2,(H2,24,28)(H,25,26)/t14?,15?,16?,17-,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329313

((S)-3-(3-Trifluoromethyl-pyridin-2-ylamino)-pyrrol...)Show SMILES NC(=O)[C@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C(F)(F)F)C(C3)C2 |r,wD:16.19,3.2,9.10,TLB:30:29:4.5.6:8,THB:30:5:8:31.29.9,9:29:4:6.7.8,9:7:4:31.30.29,10:9:4.5.6:8,(23.92,-31.27,;25.27,-30.5,;25.27,-28.96,;26.61,-31.27,;25.41,-32.55,;26.91,-32.13,;26.91,-30.55,;27.95,-29.31,;26.6,-29.79,;29.34,-29.89,;30.68,-29.13,;32.01,-29.91,;32,-31.45,;33.35,-29.16,;33.38,-27.61,;34.85,-27.16,;35.74,-28.42,;34.81,-29.65,;37.28,-28.44,;38.07,-27.12,;37.32,-25.79,;38.1,-24.47,;39.65,-24.49,;40.4,-25.84,;39.61,-27.16,;40.35,-28.5,;39.56,-29.82,;41.89,-28.53,;41.11,-29.83,;29.33,-31.42,;28.32,-32.7,;27.94,-31.76,)| Show InChI InChI=1S/C22H27F3N4O3/c23-22(24,25)16-2-1-4-27-18(16)28-15-3-5-29(11-15)20(31)32-17-13-6-12-7-14(17)10-21(8-12,9-13)19(26)30/h1-2,4,12-15,17H,3,5-11H2,(H2,26,30)(H,27,28)/t12?,13?,14?,15-,17-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329317

((S)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(2.39,-49.17,;3.73,-48.39,;3.74,-46.85,;5.07,-49.17,;3.88,-50.45,;5.38,-50.03,;6.78,-50.59,;7.8,-49.31,;6.4,-49.66,;7.81,-47.79,;9.15,-47.03,;10.47,-47.81,;10.46,-49.35,;11.82,-47.05,;11.84,-45.51,;13.31,-45.06,;14.2,-46.32,;13.27,-47.55,;15.74,-46.34,;16.53,-45.02,;15.78,-43.69,;16.56,-42.36,;18.11,-42.39,;18.86,-43.73,;18.07,-45.05,;18.82,-46.4,;19.56,-47.74,;6.41,-47.21,;5.37,-48.44,;5.06,-47.69,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329319

(Adamantan-2-ol | CHEMBL1269176)Show SMILES OC1C2CC3CC(C2)CC1C3 |TLB:7:6:10:3.2.1,7:2:5.6.8:10,THB:1:2:5:8.9.10,1:9:5:3.7.2,0:1:5.6.8:10,(32.02,1.86,;30.67,1.1,;30.65,-.44,;29.25,-.79,;27.91,-.3,;26.7,-1.58,;28.22,-1.16,;29.63,-1.73,;28.21,.44,;29.26,1.68,;27.9,1.2,)| Show InChI InChI=1S/C10H16O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-11H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50329315

((R)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:16.19,9.10,wD:3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(3.85,-46.65,;5.2,-45.88,;5.2,-44.34,;6.53,-46.66,;5.34,-47.93,;6.84,-47.51,;8.24,-48.08,;9.26,-46.8,;7.86,-47.15,;9.27,-45.27,;10.61,-44.52,;11.94,-45.3,;11.92,-46.84,;13.28,-44.54,;13.3,-43,;14.78,-42.54,;15.66,-43.8,;14.74,-45.03,;17.2,-43.83,;17.99,-42.51,;17.24,-41.17,;18.03,-39.85,;19.58,-39.87,;20.32,-41.22,;19.53,-42.54,;20.28,-43.88,;21.03,-45.23,;7.87,-44.69,;6.83,-45.93,;6.53,-45.17,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50329288

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(33.72,4.21,;32.18,4.23,;31.44,5.58,;32.95,5.58,;31.39,2.91,;29.85,2.94,;29.11,4.28,;29.06,1.62,;27.52,1.64,;26.64,2.9,;25.17,2.45,;25.14,.9,;26.6,.41,;23.8,.15,;23.78,-1.39,;22.47,.93,;21.13,.17,;21.12,-1.36,;19.72,-1.7,;18.4,-1.21,;17.2,-2.49,;18.7,-2.07,;20.11,-2.63,;18.7,-.49,;19.73,.75,;18.39,.27,)| Show InChI InChI=1S/C20H32N2O4/c1-20(2,3)26-18(23)21-16-4-5-22(11-16)19(24)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329319

(Adamantan-2-ol | CHEMBL1269176)Show SMILES OC1C2CC3CC(C2)CC1C3 |TLB:7:6:10:3.2.1,7:2:5.6.8:10,THB:1:2:5:8.9.10,1:9:5:3.7.2,0:1:5.6.8:10,(32.02,1.86,;30.67,1.1,;30.65,-.44,;29.25,-.79,;27.91,-.3,;26.7,-1.58,;28.22,-1.16,;29.63,-1.73,;28.21,.44,;29.26,1.68,;27.9,1.2,)| Show InChI InChI=1S/C10H16O/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-11H,1-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329288

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:8.7,TLB:22:21:25:18.17.16,22:17:20.21.23:25,THB:16:17:20:23.24.25,16:24:20:18.22.17,15:16:20.21.23:25,(33.72,4.21,;32.18,4.23,;31.44,5.58,;32.95,5.58,;31.39,2.91,;29.85,2.94,;29.11,4.28,;29.06,1.62,;27.52,1.64,;26.64,2.9,;25.17,2.45,;25.14,.9,;26.6,.41,;23.8,.15,;23.78,-1.39,;22.47,.93,;21.13,.17,;21.12,-1.36,;19.72,-1.7,;18.4,-1.21,;17.2,-2.49,;18.7,-2.07,;20.11,-2.63,;18.7,-.49,;19.73,.75,;18.39,.27,)| Show InChI InChI=1S/C20H32N2O4/c1-20(2,3)26-18(23)21-16-4-5-22(11-16)19(24)25-17-14-7-12-6-13(9-14)10-15(17)8-12/h12-17H,4-11H2,1-3H3,(H,21,23)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50329317

((S)-3-(3-Cyano-pyridin-2-ylamino)-pyrrolidine-1-ca...)Show SMILES NC(=O)[C@@]12CC3CC(C1)[C@H](OC(=O)N1CC[C@@H](C1)Nc1ncccc1C#N)C(C3)C2 |r,wU:9.10,wD:16.19,3.2,TLB:6:5:29:8.7.9,6:7:4.5.28:29,THB:9:7:4:28.27.29,9:27:4:8.6.7,10:9:4.5.28:29,(2.39,-49.17,;3.73,-48.39,;3.74,-46.85,;5.07,-49.17,;3.88,-50.45,;5.38,-50.03,;6.78,-50.59,;7.8,-49.31,;6.4,-49.66,;7.81,-47.79,;9.15,-47.03,;10.47,-47.81,;10.46,-49.35,;11.82,-47.05,;11.84,-45.51,;13.31,-45.06,;14.2,-46.32,;13.27,-47.55,;15.74,-46.34,;16.53,-45.02,;15.78,-43.69,;16.56,-42.36,;18.11,-42.39,;18.86,-43.73,;18.07,-45.05,;18.82,-46.4,;19.56,-47.74,;6.41,-47.21,;5.37,-48.44,;5.06,-47.69,)| Show InChI InChI=1S/C22H27N5O3/c23-11-14-2-1-4-25-19(14)26-17-3-5-27(12-17)21(29)30-18-15-6-13-7-16(18)10-22(8-13,9-15)20(24)28/h1-2,4,13,15-18H,3,5-10,12H2,(H2,24,28)(H,25,26)/t13?,15?,16?,17-,18-,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity against GR |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50329302

((R)-3-tert-Butoxycarbonylamino-pyrrolidine-1-carbo...)Show SMILES CC(C)(C)OC(=O)N[C@@H]1CCN(C1)C(=O)O[C@H]1C2CC3CC1C[C@](C3)(C2)C(N)=O |r,wU:8.7,16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:19:22:25.17.16,18:17:24.19.20:22,16:21:24:25.18.17,(42.66,-42.33,;41.12,-42.3,;40.37,-40.95,;41.88,-40.96,;40.33,-43.62,;38.79,-43.6,;38.04,-42.25,;38,-44.92,;36.46,-44.89,;35.57,-43.63,;34.1,-44.08,;34.08,-45.63,;35.53,-46.12,;32.73,-46.39,;32.72,-47.93,;31.41,-45.61,;30.07,-46.36,;30.06,-47.89,;29.04,-49.17,;27.64,-48.6,;27.63,-47.02,;28.67,-45.78,;27.32,-46.26,;27.33,-47.75,;26.14,-49.02,;28.66,-48.24,;25.99,-46.97,;24.65,-47.74,;26,-45.43,)| Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)29-18(26)23-15-4-5-24(11-15)19(27)28-16-13-6-12-7-14(16)10-21(8-12,9-13)17(22)25/h12-16H,4-11H2,1-3H3,(H2,22,25)(H,23,26)/t12?,13?,14?,15-,16-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activity against FXR |
Bioorg Med Chem Lett 20: 6725-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.142
BindingDB Entry DOI: 10.7270/Q2X92BJ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data