

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659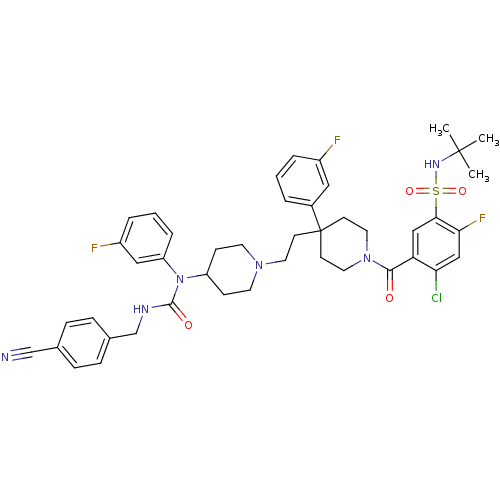 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331658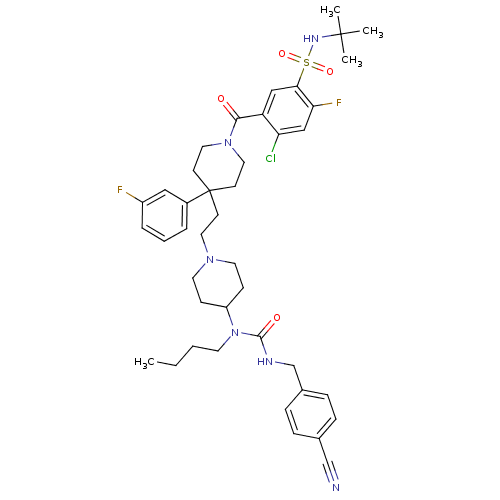 (CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331651 (CHEMBL1288917 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646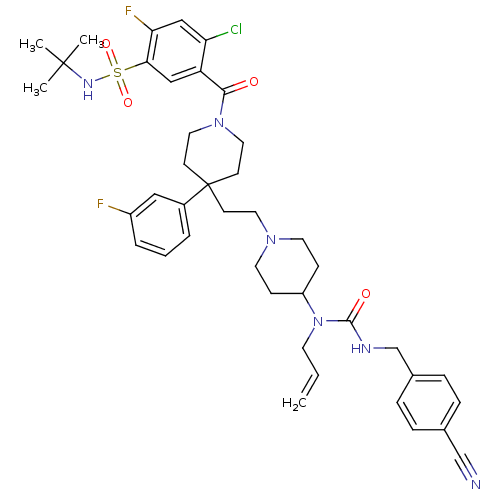 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331638 (4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331639 (4-cyanobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331642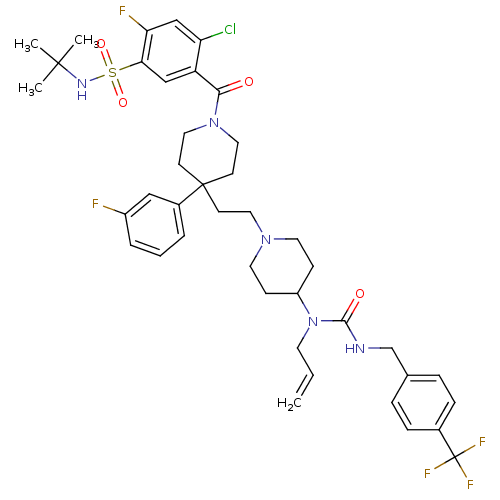 (5-(4-(2-(4-(1-allyl-3-(4-(trifluoromethyl)benzyl)u...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331660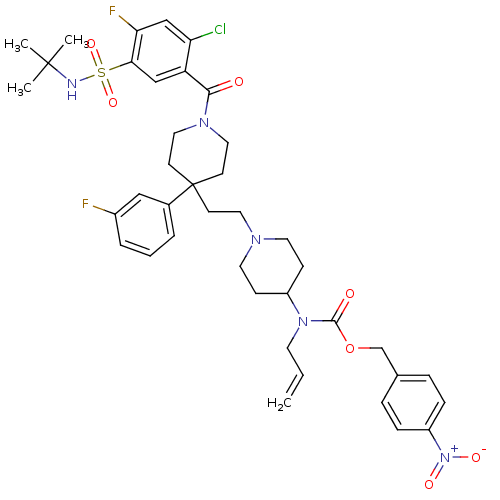 (4-nitrobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331658 (CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331647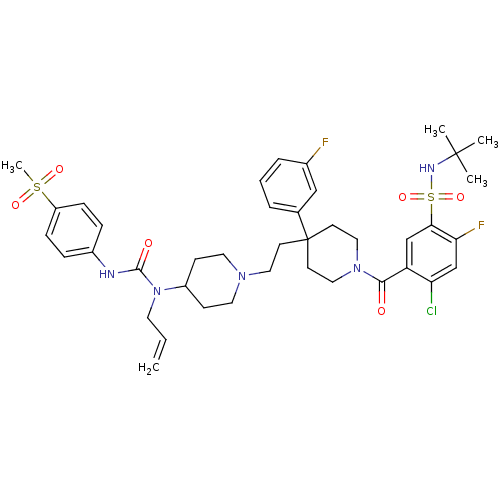 (5-(4-(2-(4-(1-allyl-3-(4-(methylsulfonyl)phenyl)ur...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331661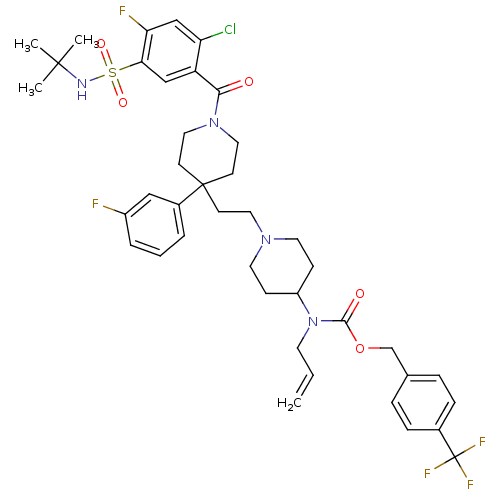 (4-(trifluoromethyl)benzyl allyl(1-(2-(1-(5-(N-tert...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331638 (4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331654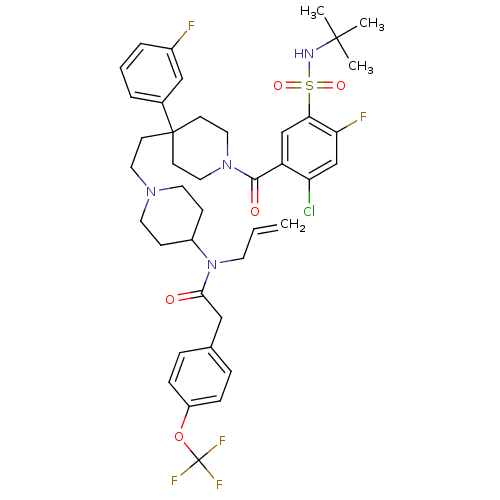 (CHEMBL1288920 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331645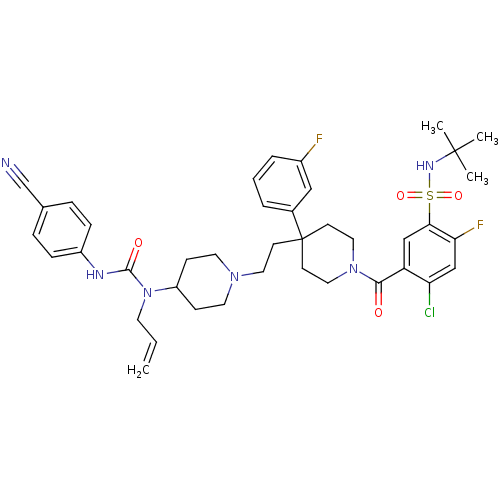 (5-(4-(2-(4-(1-allyl-3-(4-cyanophenyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331662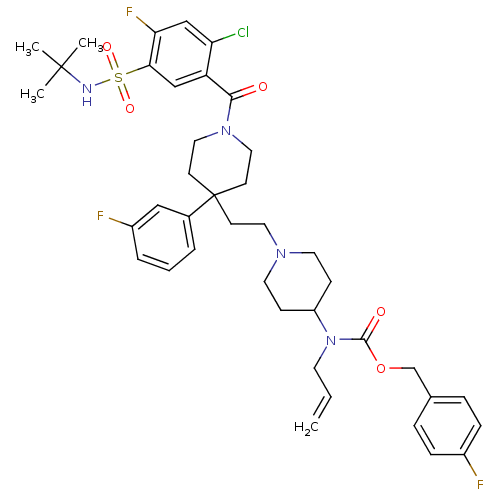 (4-fluorobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331660 (4-nitrobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331645 (5-(4-(2-(4-(1-allyl-3-(4-cyanophenyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331648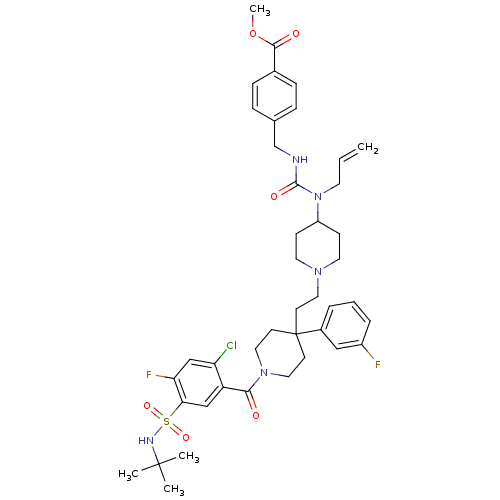 (CHEMBL1288914 | methyl 4-((3-allyl-3-(1-(2-(1-(5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331640 (4-(trifluoromethoxy)benzyl allyl(1-(2-(1-(5-(N-ter...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331643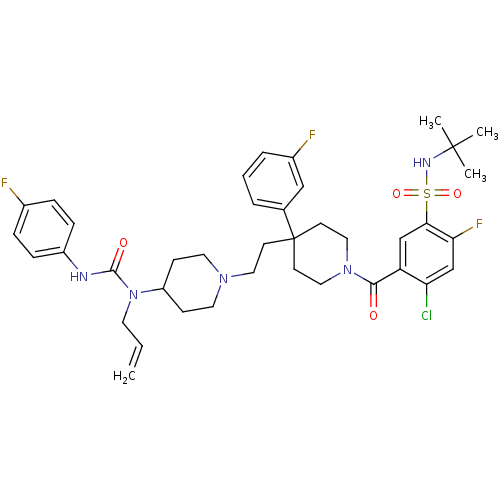 (5-(4-(2-(4-(1-allyl-3-(4-fluorophenyl)ureido)piper...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331647 (5-(4-(2-(4-(1-allyl-3-(4-(methylsulfonyl)phenyl)ur...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331661 (4-(trifluoromethyl)benzyl allyl(1-(2-(1-(5-(N-tert...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331663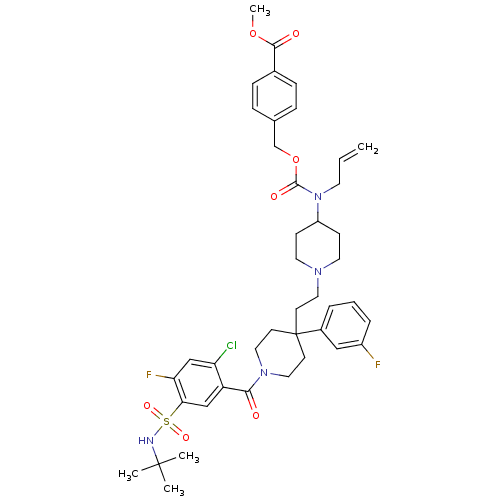 (CHEMBL1288875 | methyl 4-((allyl(1-(2-(1-(5-(N-ter...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331642 (5-(4-(2-(4-(1-allyl-3-(4-(trifluoromethyl)benzyl)u...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331644 (5-(4-(2-(4-(1-allyl-3-(4-fluorobenzyl)ureido)piper...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331652 (CHEMBL1288918 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331651 (CHEMBL1288917 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331650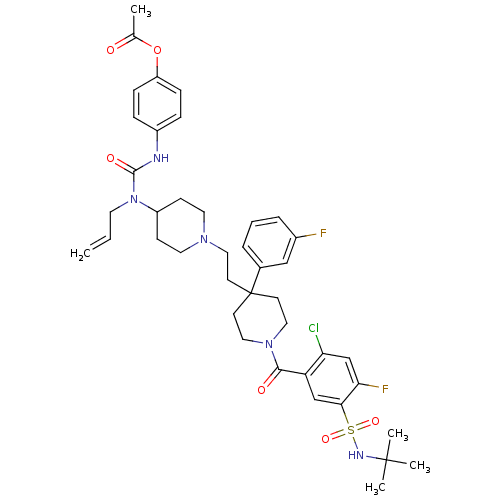 (4-(3-allyl-3-(1-(2-(1-(5-(N-tert-butylsulfamoyl)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331641 (5-(4-(2-(4-(1-allyl-3-(4-(trifluoromethyl)phenyl)u...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331653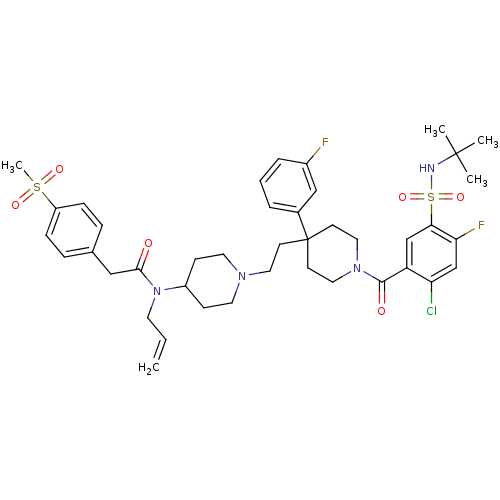 (CHEMBL1288919 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331654 (CHEMBL1288920 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331648 (CHEMBL1288914 | methyl 4-((3-allyl-3-(1-(2-(1-(5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331657 (CHEMBL1288923 | N-allyl-N-(1-(2-(1-(2-chloro-4-flu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331655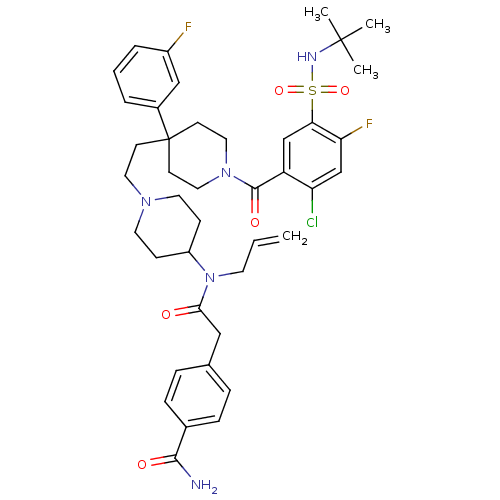 (4-(2-(allyl(1-(2-(1-(5-(N-tert-butylsulfamoyl)-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331656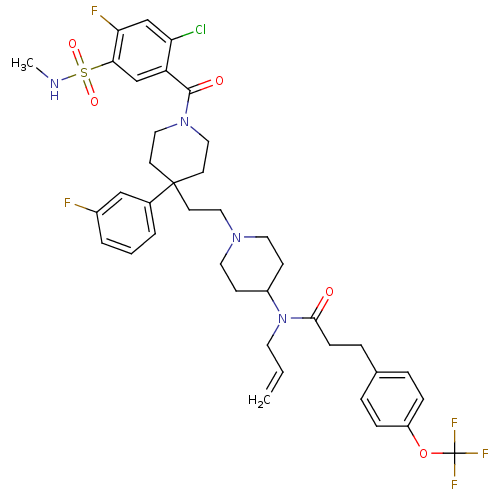 (CHEMBL1288922 | N-allyl-N-(1-(2-(1-(2-chloro-4-flu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331649 (4-((3-allyl-3-(1-(2-(1-(5-(N-tert-butylsulfamoyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||