| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50331654 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_687623 (CHEMBL1290947) |
|---|
| IC50 | 87±n/a nM |
|---|
| Citation |  Duan, M; Peckham, J; Edelstein, M; Ferris, R; Kazmierski, WM; Spaltenstein, A; Wheelan, P; Xiong, Z Discovery of N-benzyl-N'-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (I): optimization of the amine portion. Bioorg Med Chem Lett20:7397-400 (2010) [PubMed] Article Duan, M; Peckham, J; Edelstein, M; Ferris, R; Kazmierski, WM; Spaltenstein, A; Wheelan, P; Xiong, Z Discovery of N-benzyl-N'-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (I): optimization of the amine portion. Bioorg Med Chem Lett20:7397-400 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50331654 |
|---|
| n/a |
|---|
| Name | BDBM50331654 |
|---|
| Synonyms: | CHEMBL1288920 | N-allyl-N-(1-(2-(1-(5-(N-tert-butylsulfamoyl)-2-chloro-4-fluorobenzoyl)-4-(3-fluorophenyl)piperidin-4-yl)ethyl)piperidin-4-yl)-2-(4-(trifluoromethoxy)phenyl)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C41H48ClF5N4O5S |
|---|
| Mol. Mass. | 839.354 |
|---|
| SMILES | CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)Cc3ccc(OC(F)(F)F)cc3)(CC2)c2cccc(F)c2)c(Cl)cc1F |
|---|
| Structure | 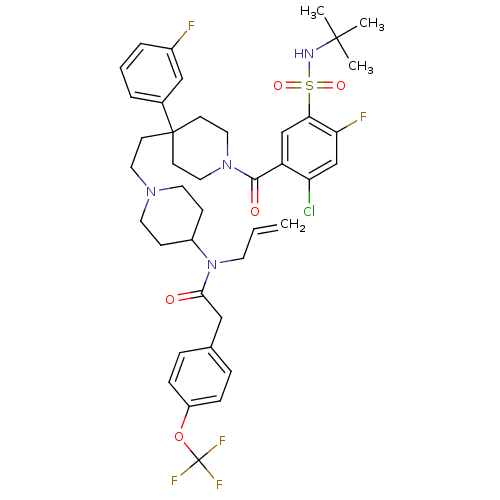
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Duan, M; Peckham, J; Edelstein, M; Ferris, R; Kazmierski, WM; Spaltenstein, A; Wheelan, P; Xiong, Z Discovery of N-benzyl-N'-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (I): optimization of the amine portion. Bioorg Med Chem Lett20:7397-400 (2010) [PubMed] Article
Duan, M; Peckham, J; Edelstein, M; Ferris, R; Kazmierski, WM; Spaltenstein, A; Wheelan, P; Xiong, Z Discovery of N-benzyl-N'-(4-pipyridinyl)urea CCR5 antagonists as anti-HIV-1 agents (I): optimization of the amine portion. Bioorg Med Chem Lett20:7397-400 (2010) [PubMed] Article