 Found 344 hits with Last Name = 'duan' and Initial = 'm'
Found 344 hits with Last Name = 'duan' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM636848
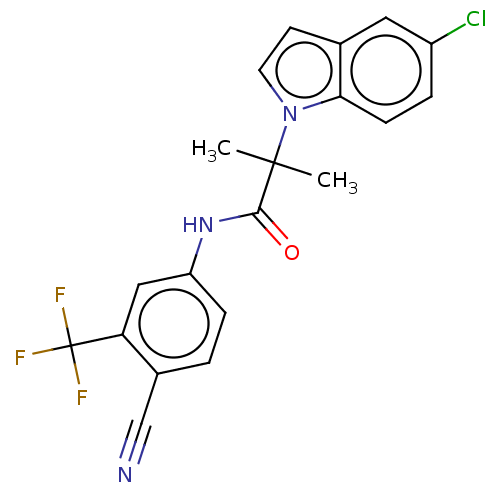
(US20230382870, Compound of Example 7)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2cc(Cl)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636846
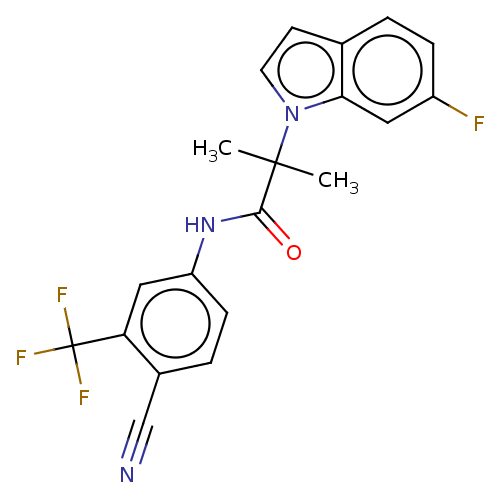
(US20230382870, Compound of Example 5)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2ccc(F)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636853
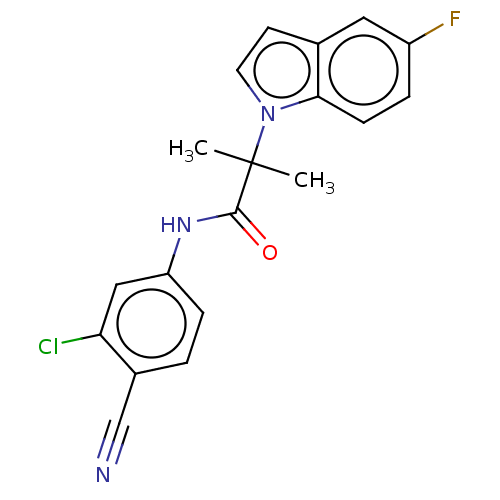
(US20230382870, Compound of Example 12)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(Cl)c1)n1ccc2cc(F)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636844
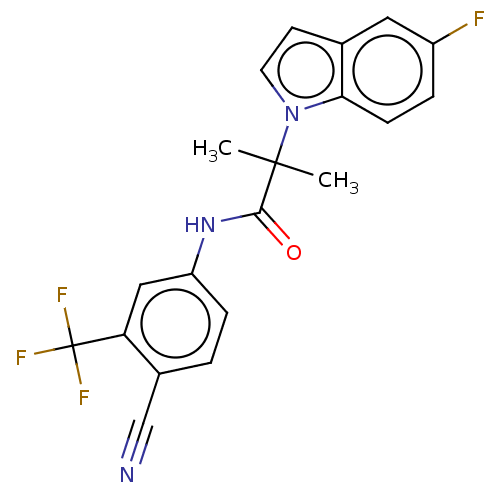
(US20230382870, Compound of Example 3)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2cc(F)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636851
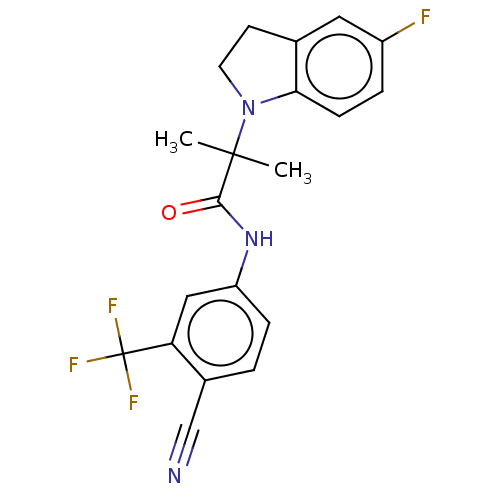
(US20230382870, Compound of Example 10)Show SMILES CC(C)(N1CCc2cc(F)ccc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636847
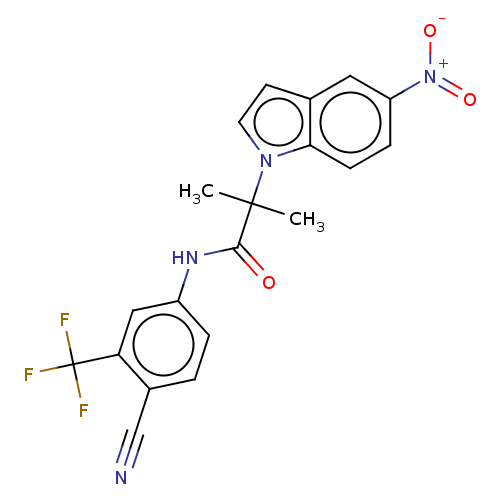
(US20230382870, Compound of Example 6)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2cc(ccc12)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 421 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636849
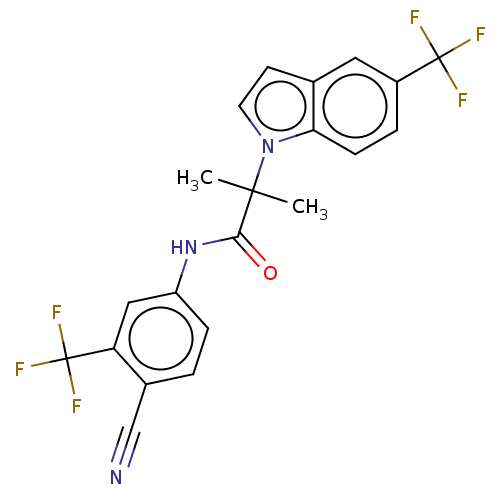
(US20230382870, Compound of Example 8)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2cc(ccc12)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636850
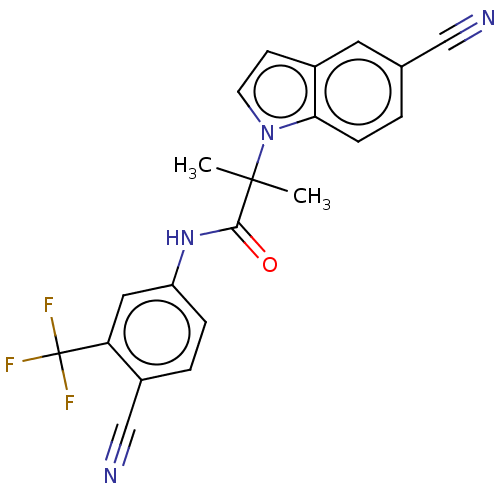
(US20230382870, Compound of Example 9)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2cc(ccc12)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636845
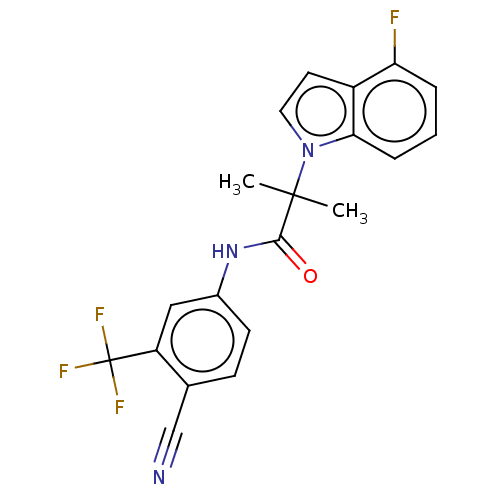
(US20230382870, Compound of Example 4)Show SMILES CC(C)(C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F)n1ccc2c(F)cccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 491 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM636852
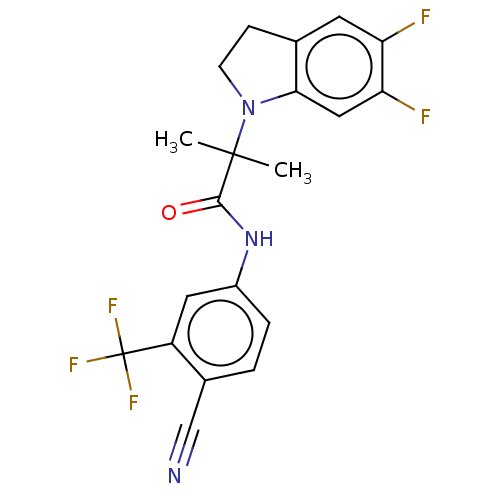
(US20230382870, Compound of Example 11)Show SMILES CC(C)(N1CCc2cc(F)c(F)cc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18678
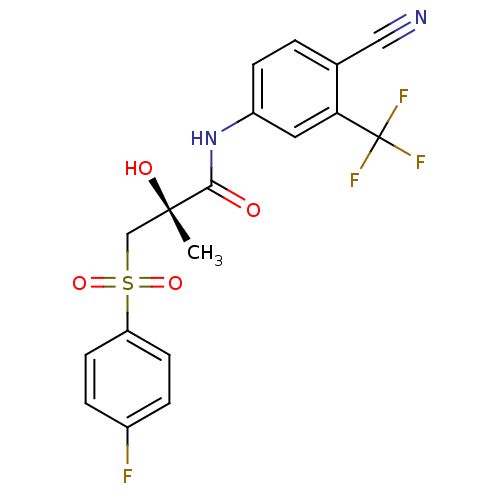
((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
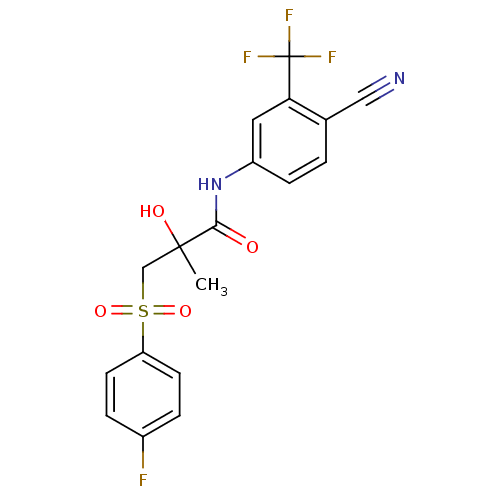
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50425732
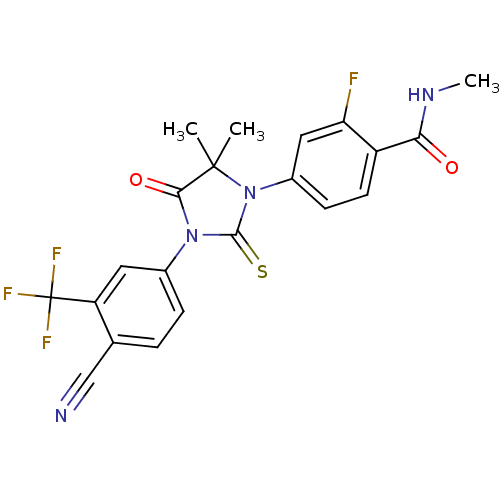
(ENZALUTAMIDE | US10053433, FC 4.129 | US10806720, ...)Show SMILES CNC(=O)c1ccc(cc1F)N1C(=S)N(C(=O)C1(C)C)c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F4N4O2S/c1-20(2)18(31)28(12-5-4-11(10-26)15(8-12)21(23,24)25)19(32)29(20)13-6-7-14(16(22)9-13)17(30)27-3/h4-9H,1-3H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
| 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595239
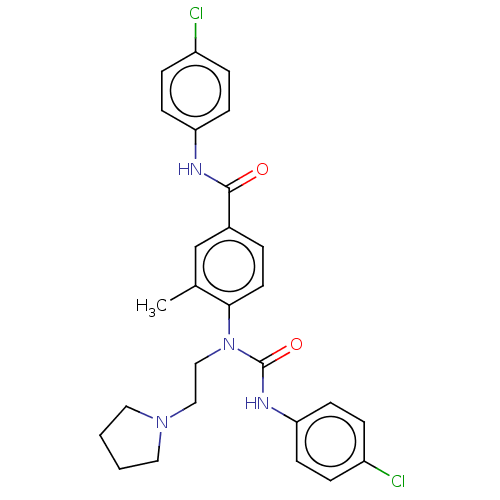
(CHEMBL5190894)Show SMILES Cc1cc(ccc1N(CCN1CCCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595236
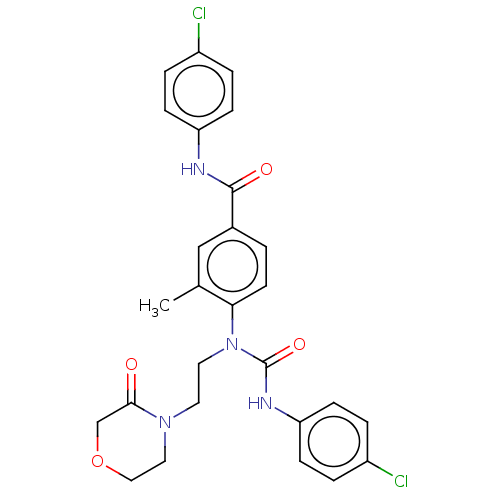
(CHEMBL5170467)Show SMILES Cc1cc(ccc1N(CCN1CCOCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595244
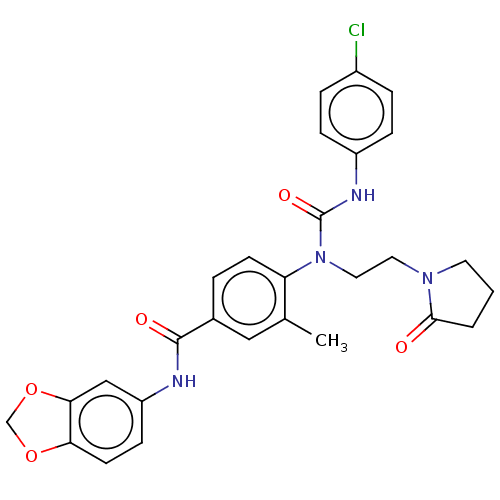
(CHEMBL5199526)Show SMILES Cc1cc(ccc1N(CCN1CCCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc2OCOc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50418490
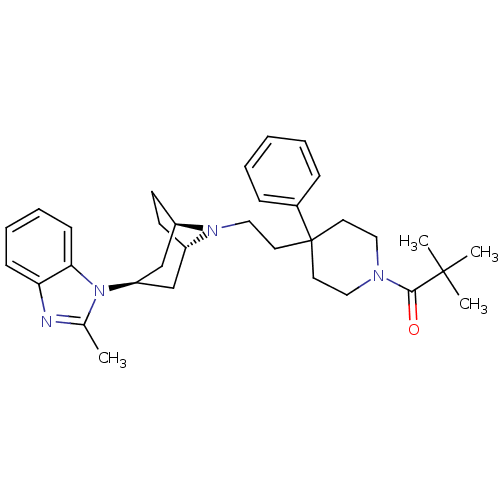
(CHEMBL1784385)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)C(C)(C)C)c1ccccc1 |r,THB:18:17:11.10.16:13.14| Show InChI InChI=1S/C33H44N4O/c1-24-34-29-12-8-9-13-30(29)37(24)28-22-26-14-15-27(23-28)36(26)21-18-33(25-10-6-5-7-11-25)16-19-35(20-17-33)31(38)32(2,3)4/h5-13,26-28H,14-23H2,1-4H3/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting |
J Med Chem 54: 3756-67 (2011)
Article DOI: 10.1021/jm200279v
BindingDB Entry DOI: 10.7270/Q26111K7 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331659
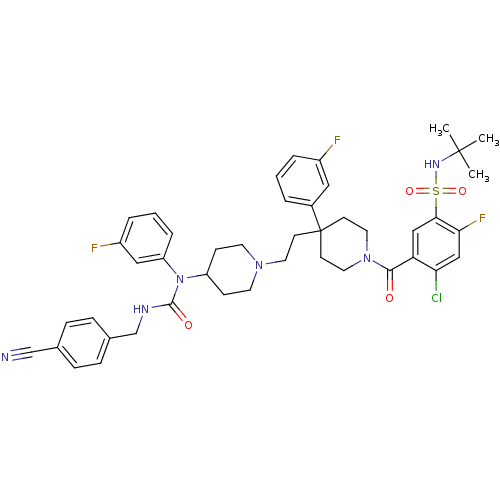
(CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(C(=O)NCc3ccc(cc3)C#N)c3cccc(F)c3)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C44H48ClF3N6O4S/c1-43(2,3)51-59(57,58)40-26-37(38(45)27-39(40)48)41(55)53-22-17-44(18-23-53,32-6-4-7-33(46)24-32)16-21-52-19-14-35(15-20-52)54(36-9-5-8-34(47)25-36)42(56)50-29-31-12-10-30(28-49)11-13-31/h4-13,24-27,35,51H,14-23,29H2,1-3H3,(H,50,56) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595240

(CHEMBL5202839)Show SMILES Cc1cc(ccc1N(CCN1CCCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at glucocorticoid receptor in human HeLa cells assessed as reduction in dexamethasone-induced luciferase activity by dual-Glo luc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01287
BindingDB Entry DOI: 10.7270/Q2VT1X06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595242
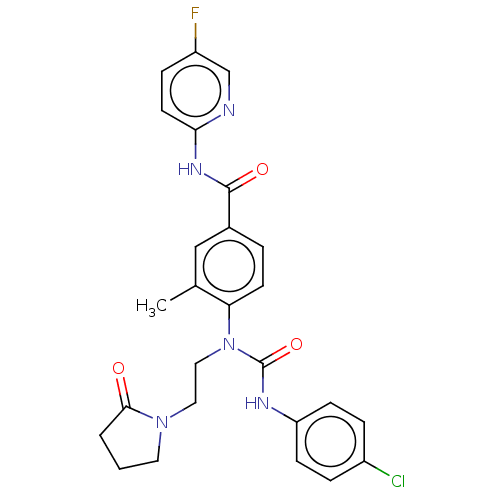
(CHEMBL5173452)Show SMILES Cc1cc(ccc1N(CCN1CCCC1=O)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(F)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50217448
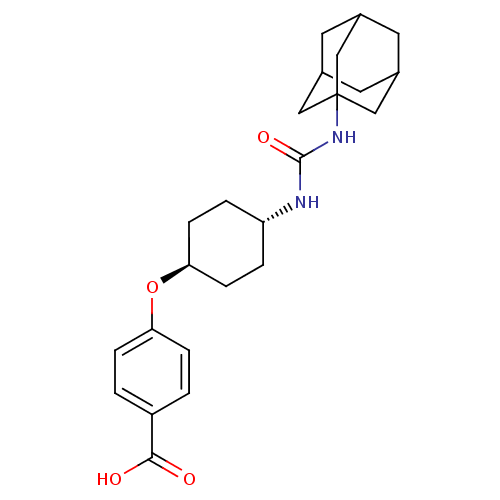
(CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...)Show SMILES OC(=O)c1ccc(O[C@H]2CC[C@@H](CC2)NC(=O)NC23CC4CC(CC(C4)C2)C3)cc1 |wU:8.7,wD:11.14,TLB:17:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18,17:18:21:25.24.23,(19.63,-26.42,;18.81,-25.12,;19.53,-23.76,;17.27,-25.17,;16.54,-26.54,;15.01,-26.59,;14.19,-25.28,;12.65,-25.34,;11.93,-26.7,;12.75,-28.01,;12.03,-29.36,;10.48,-29.42,;9.67,-28.12,;10.39,-26.76,;9.77,-30.78,;8.23,-30.84,;7.41,-29.54,;7.51,-32.21,;5.97,-32.27,;4.96,-33.54,;3.55,-32.98,;2.06,-33.4,;3.25,-32.13,;3.24,-30.64,;4.59,-30.16,;3.55,-31.39,;5.99,-30.74,;4.58,-32.61,;14.91,-23.93,;16.44,-23.87,)| Show InChI InChI=1S/C24H32N2O4/c27-22(28)18-1-5-20(6-2-18)30-21-7-3-19(4-8-21)25-23(29)26-24-12-15-9-16(13-24)11-17(10-15)14-24/h1-2,5-6,15-17,19,21H,3-4,7-14H2,(H,27,28)(H2,25,26,29)/t15?,16?,17?,19-,21-,24? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458067
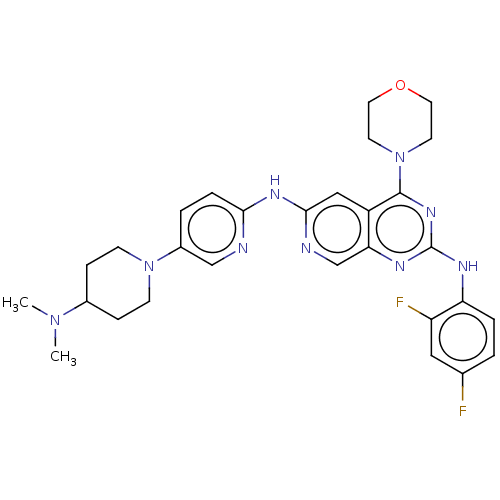
(CHEMBL4215080)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4F)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H33F2N9O/c1-38(2)20-7-9-39(10-8-20)21-4-6-26(32-17-21)36-27-16-22-25(18-33-27)35-29(34-24-5-3-19(30)15-23(24)31)37-28(22)40-11-13-41-14-12-40/h3-6,15-18,20H,7-14H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595230
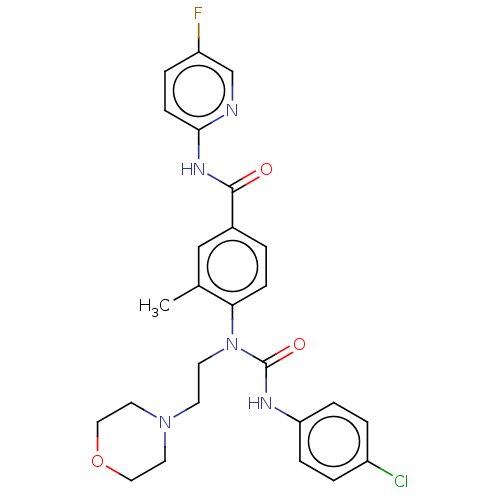
(CHEMBL5201407)Show SMILES Cc1cc(ccc1N(CCN1CCOCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(F)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257737
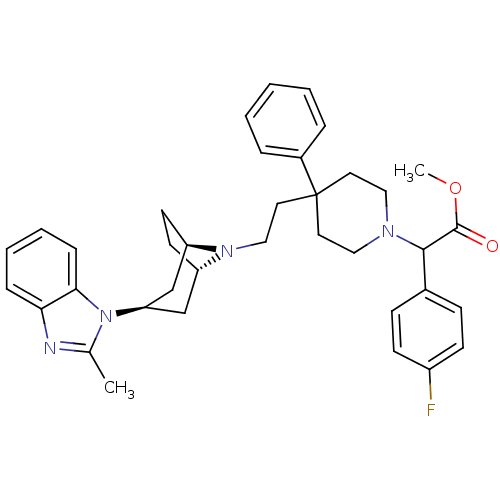
(CHEMBL444497 | methyl 2-(4-fluorophenyl)-2-(4-(2-(...)Show SMILES COC(=O)C(N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1)c1ccc(F)cc1 |r,THB:19:17:11:13.14| Show InChI InChI=1S/C37H43FN4O2/c1-26-39-33-10-6-7-11-34(33)42(26)32-24-30-16-17-31(25-32)41(30)23-20-37(28-8-4-3-5-9-28)18-21-40(22-19-37)35(36(43)44-2)27-12-14-29(38)15-13-27/h3-15,30-32,35H,16-25H2,1-2H3/t30-,31+,32+,35? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595241
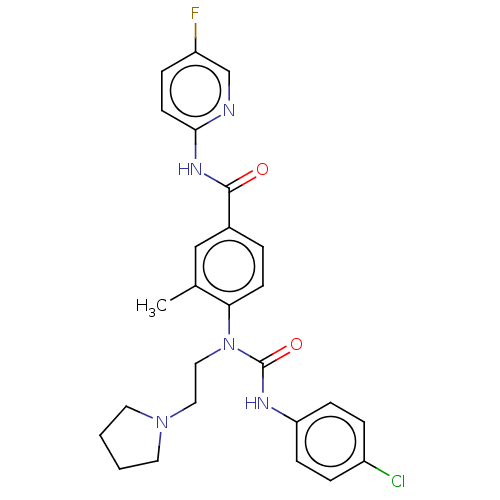
(CHEMBL5203957)Show SMILES Cc1cc(ccc1N(CCN1CCCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(F)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458066
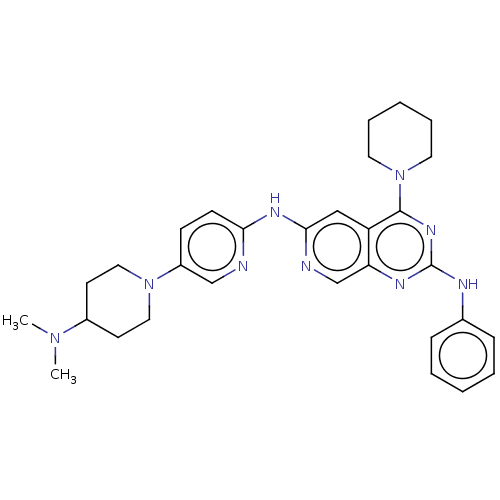
(CHEMBL4203016)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C30H37N9/c1-37(2)23-13-17-38(18-14-23)24-11-12-27(31-20-24)35-28-19-25-26(21-32-28)34-30(33-22-9-5-3-6-10-22)36-29(25)39-15-7-4-8-16-39/h3,5-6,9-12,19-21,23H,4,7-8,13-18H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595232
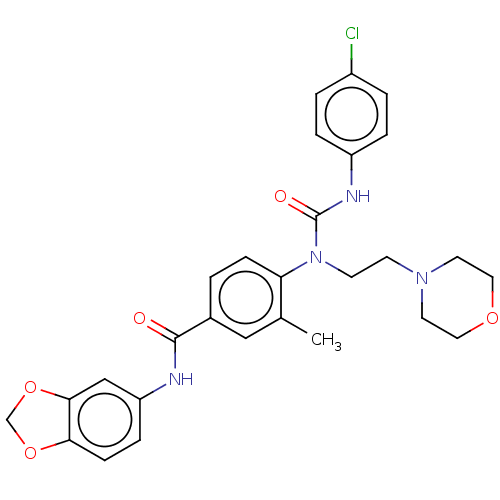
(CHEMBL5189164)Show SMILES Cc1cc(ccc1N(CCN1CCOCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc2OCOc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068
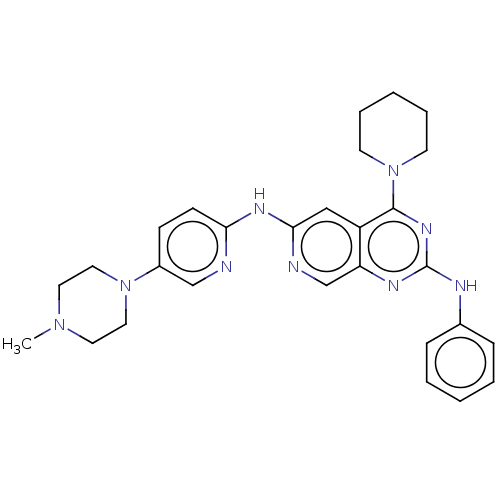
(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458062
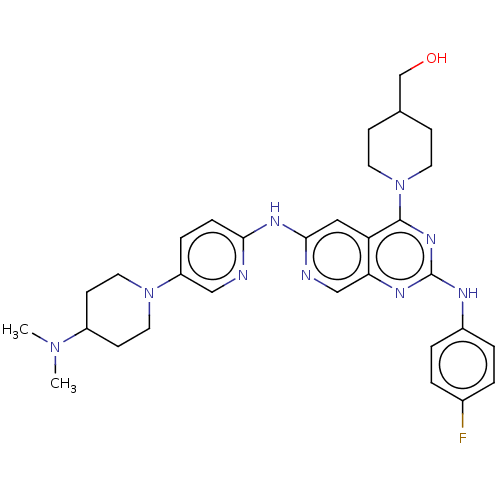
(CHEMBL4209019)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCC(CO)CC2)nc1 Show InChI InChI=1S/C31H38FN9O/c1-39(2)24-11-15-40(16-12-24)25-7-8-28(33-18-25)37-29-17-26-27(19-34-29)36-31(35-23-5-3-22(32)4-6-23)38-30(26)41-13-9-21(20-42)10-14-41/h3-8,17-19,21,24,42H,9-16,20H2,1-2H3,(H,33,34,37)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458068

(CHEMBL4206716)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCCC2)nc1 Show InChI InChI=1S/C28H33N9/c1-35-14-16-36(17-15-35)22-10-11-25(29-19-22)33-26-18-23-24(20-30-26)32-28(31-21-8-4-2-5-9-21)34-27(23)37-12-6-3-7-13-37/h2,4-5,8-11,18-20H,3,6-7,12-17H2,1H3,(H,29,30,33)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458060
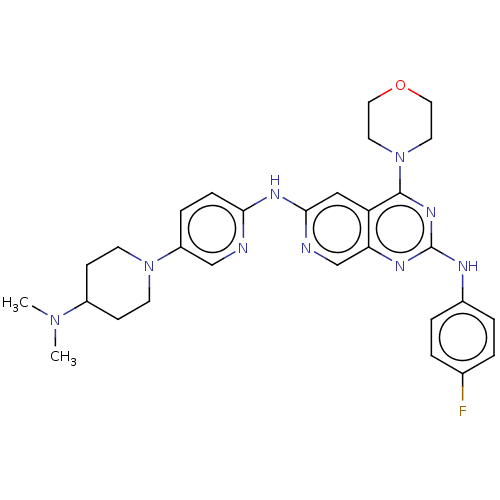
(CHEMBL4215076)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H34FN9O/c1-37(2)22-9-11-38(12-10-22)23-7-8-26(31-18-23)35-27-17-24-25(19-32-27)34-29(33-21-5-3-20(30)4-6-21)36-28(24)39-13-15-40-16-14-39/h3-8,17-19,22H,9-16H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458065
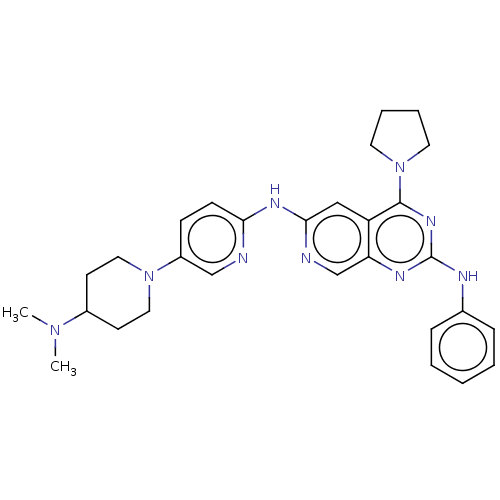
(CHEMBL4212884)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCC2)nc1 Show InChI InChI=1S/C29H35N9/c1-36(2)22-12-16-37(17-13-22)23-10-11-26(30-19-23)34-27-18-24-25(20-31-27)33-29(32-21-8-4-3-5-9-21)35-28(24)38-14-6-7-15-38/h3-5,8-11,18-20,22H,6-7,12-17H2,1-2H3,(H,30,31,34)(H,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331659

(CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(C(=O)NCc3ccc(cc3)C#N)c3cccc(F)c3)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C44H48ClF3N6O4S/c1-43(2,3)51-59(57,58)40-26-37(38(45)27-39(40)48)41(55)53-22-17-44(18-23-53,32-6-4-7-33(46)24-32)16-21-52-19-14-35(15-20-52)54(36-9-5-8-34(47)25-36)42(56)50-29-31-12-10-30(28-49)11-13-31/h4-13,24-27,35,51H,14-23,29H2,1-3H3,(H,50,56) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595237
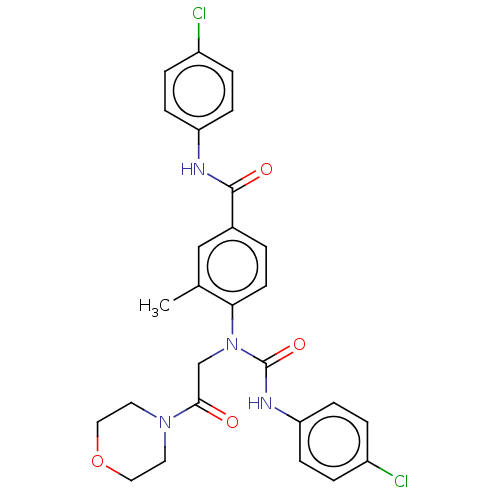
(CHEMBL5203928)Show SMILES Cc1cc(ccc1N(CC(=O)N1CCOCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257690
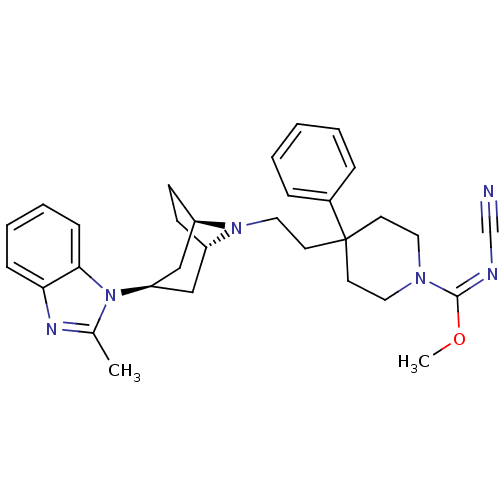
((E)-methyl N-cyano-4-(2-(3-(2-methyl-1H-benzo[d]im...)Show SMILES CO\C(=N\C#N)N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1 |r,THB:20:18:12:14.15| Show InChI InChI=1S/C31H38N6O/c1-23-34-28-10-6-7-11-29(28)37(23)27-20-25-12-13-26(21-27)36(25)19-16-31(24-8-4-3-5-9-24)14-17-35(18-15-31)30(38-2)33-22-32/h3-11,25-27H,12-21H2,1-2H3/b33-30+/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458058
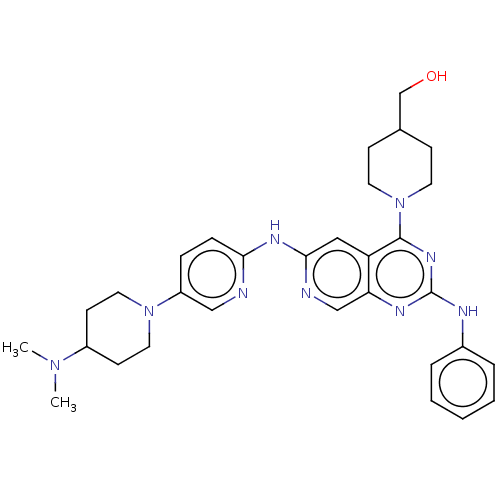
(CHEMBL4206166)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCC(CO)CC2)nc1 Show InChI InChI=1S/C31H39N9O/c1-38(2)24-12-16-39(17-13-24)25-8-9-28(32-19-25)36-29-18-26-27(20-33-29)35-31(34-23-6-4-3-5-7-23)37-30(26)40-14-10-22(21-41)11-15-40/h3-9,18-20,22,24,41H,10-17,21H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458067

(CHEMBL4215080)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4F)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H33F2N9O/c1-38(2)20-7-9-39(10-8-20)21-4-6-26(32-17-21)36-27-16-22-25(18-33-27)35-29(34-24-5-3-19(30)15-23(24)31)37-28(22)40-11-13-41-14-12-40/h3-6,15-18,20H,7-14H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458063
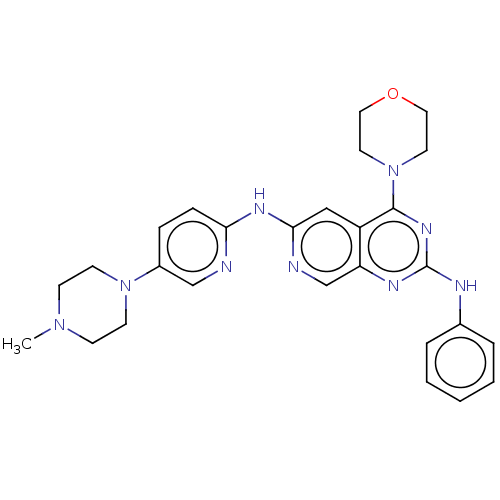
(CHEMBL4206288)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C27H31N9O/c1-34-9-11-35(12-10-34)21-7-8-24(28-18-21)32-25-17-22-23(19-29-25)31-27(30-20-5-3-2-4-6-20)33-26(22)36-13-15-37-16-14-36/h2-8,17-19H,9-16H2,1H3,(H,28,29,32)(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458059
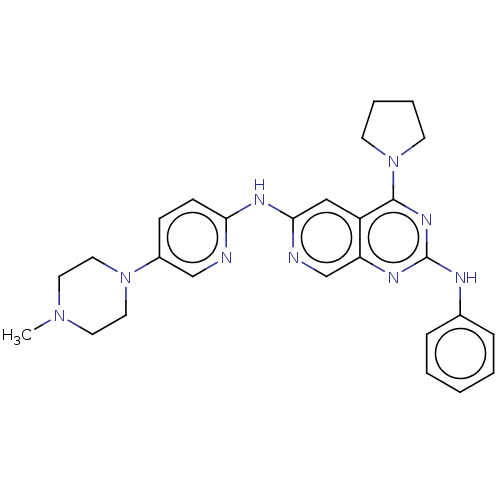
(CHEMBL4209801)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCCC2)nc1 Show InChI InChI=1S/C27H31N9/c1-34-13-15-35(16-14-34)21-9-10-24(28-18-21)32-25-17-22-23(19-29-25)31-27(30-20-7-3-2-4-8-20)33-26(22)36-11-5-6-12-36/h2-4,7-10,17-19H,5-6,11-16H2,1H3,(H,28,29,32)(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50595238
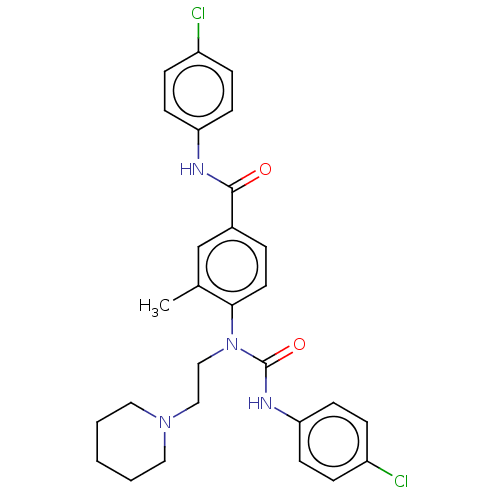
(CHEMBL5173974)Show SMILES Cc1cc(ccc1N(CCN1CCCCC1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(Cl)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| |
Bioorg Med Chem Lett 15: 3675-8 (2005)
Article DOI: 10.1016/j.bmcl.2022.128805
BindingDB Entry DOI: 10.7270/Q2J67MXV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R/T790M double mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence a... |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458060

(CHEMBL4215076)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccc(F)cc4)nc3cn2)N2CCOCC2)nc1 Show InChI InChI=1S/C29H34FN9O/c1-37(2)22-9-11-38(12-10-22)23-7-8-26(31-18-23)35-27-17-24-25(19-32-27)34-29(33-21-5-3-20(30)4-6-21)36-28(24)39-13-15-40-16-14-39/h3-8,17-19,22H,9-16H2,1-2H3,(H,31,32,35)(H,33,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331658
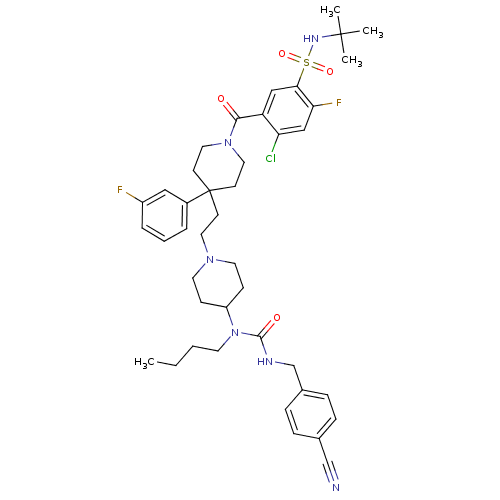
(CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...)Show SMILES CCCCN(C1CCN(CCC2(CCN(CC2)C(=O)c2cc(c(F)cc2Cl)S(=O)(=O)NC(C)(C)C)c2cccc(F)c2)CC1)C(=O)NCc1ccc(cc1)C#N Show InChI InChI=1S/C42H53ClF2N6O4S/c1-5-6-19-51(40(53)47-29-31-12-10-30(28-46)11-13-31)34-14-20-49(21-15-34)22-16-42(32-8-7-9-33(44)25-32)17-23-50(24-18-42)39(52)35-26-38(37(45)27-36(35)43)56(54,55)48-41(2,3)4/h7-13,25-27,34,48H,5-6,14-24,29H2,1-4H3,(H,47,53) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257641
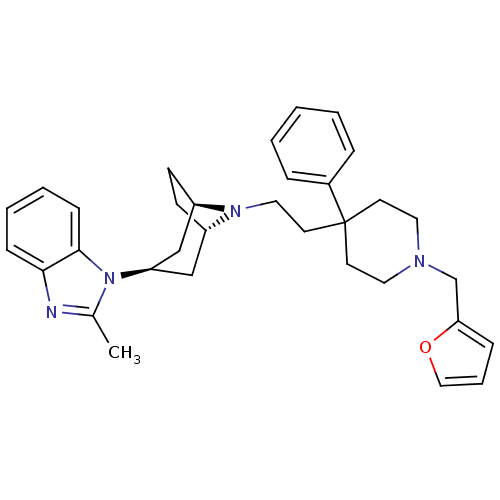
(1-(8-(2-(1-(furan-2-ylmethyl)-4-phenylpiperidin-4-...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(Cc2ccco2)CC1)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C33H40N4O/c1-25-34-31-11-5-6-12-32(31)37(25)29-22-27-13-14-28(23-29)36(27)20-17-33(26-8-3-2-4-9-26)15-18-35(19-16-33)24-30-10-7-21-38-30/h2-12,21,27-29H,13-20,22-24H2,1H3/t27-,28+,29+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257734

(2-(2,3-dimethylphenyl)-2-(4-(2-(3-(2-methyl-1H-ben...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(C(O)=O)c1cccc(C)c1C)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C38H46N4O2/c1-26-10-9-13-33(27(26)2)36(37(43)44)40-21-18-38(19-22-40,29-11-5-4-6-12-29)20-23-41-30-16-17-31(41)25-32(24-30)42-28(3)39-34-14-7-8-15-35(34)42/h4-15,30-32,36H,16-25H2,1-3H3,(H,43,44)/t30-,31+,32+,36? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257691
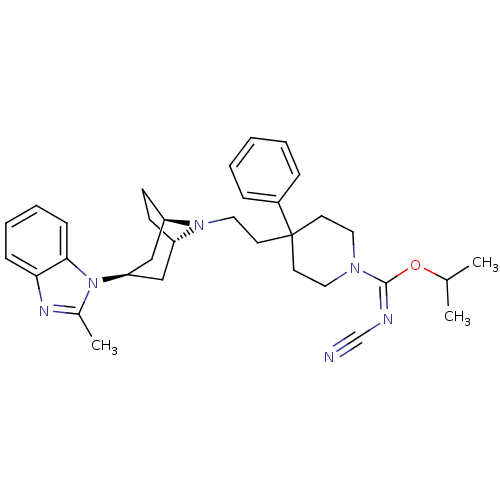
((E)-isopropyl N-cyano-4-(2-(3-(2-methyl-1H-benzo[d...)Show SMILES CC(C)O\C(=N\C#N)N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1 |r,THB:22:20:14:16.17| Show InChI InChI=1S/C33H42N6O/c1-24(2)40-32(35-23-34)37-18-15-33(16-19-37,26-9-5-4-6-10-26)17-20-38-27-13-14-28(38)22-29(21-27)39-25(3)36-30-11-7-8-12-31(30)39/h4-12,24,27-29H,13-22H2,1-3H3/b35-32+/t27-,28+,29+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50458058

(CHEMBL4206166)Show SMILES CN(C)C1CCN(CC1)c1ccc(Nc2cc3c(nc(Nc4ccccc4)nc3cn2)N2CCC(CO)CC2)nc1 Show InChI InChI=1S/C31H39N9O/c1-38(2)24-12-16-39(17-13-24)25-8-9-28(32-19-25)36-29-18-26-27(20-33-29)35-31(34-23-6-4-3-5-7-23)37-30(26)40-14-10-22(21-41)11-15-40/h3-9,18-20,22,24,41H,10-17,21H2,1-2H3,(H,32,33,36)(H,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR L858R mutant (unknown origin) using Poly (Glu, Tyr) as substrate after 40 mins by kinase-Glo luminescence assay |
Eur J Med Chem 148: 221-237 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.051
BindingDB Entry DOI: 10.7270/Q2H70JF4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data