 Found 362 hits with Last Name = 'ferris' and Initial = 'r'
Found 362 hits with Last Name = 'ferris' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM4685

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@]12OCC[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccc(OCc2csc(C)n2)cc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C33H41N3O10S2/c1-20(2)14-36(48(39,40)25-8-9-29-30(13-25)45-19-44-29)15-28(37)27(35-33(38)46-31-17-43-32-26(31)10-11-41-32)12-22-4-6-24(7-5-22)42-16-23-18-47-21(3)34-23/h4-9,13,18,20,26-28,31-32,37H,10-12,14-17,19H2,1-3H3,(H,35,38)/t26-,27-,28+,31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Antimicrob Agents Chemother 51: 3147-54 (2007)
Article DOI: 10.1128/aac.00401-07
BindingDB Entry DOI: 10.7270/Q23R0WPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by fluorescent peptide substrate based assay |
Antimicrob Agents Chemother 51: 3147-54 (2007)
Article DOI: 10.1128/aac.00401-07
BindingDB Entry DOI: 10.7270/Q23R0WPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Antimicrob Agents Chemother 51: 3147-54 (2007)
Article DOI: 10.1128/aac.00401-07
BindingDB Entry DOI: 10.7270/Q23R0WPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50479982
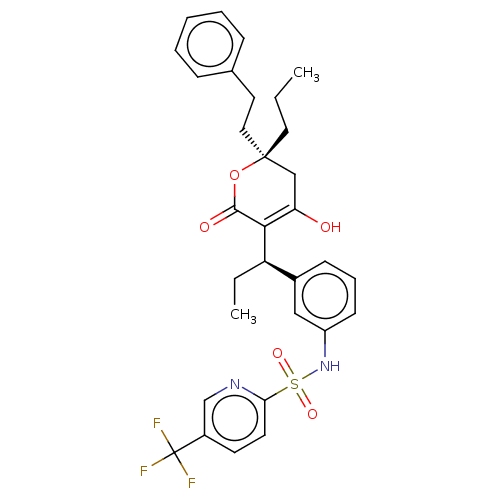
(Aptivus | CHEBI:63628 | Tipranavir | U-140690 | US...)Show SMILES CCC[C@@]1(CCc2ccccc2)CC(O)=C([C@H](CC)c2cccc(NS(=O)(=O)c3ccc(cn3)C(F)(F)F)c2)C(=O)O1 |t:15| Show InChI InChI=1S/C31H33F3N2O5S/c1-3-16-30(17-15-21-9-6-5-7-10-21)19-26(37)28(29(38)41-30)25(4-2)22-11-8-12-24(18-22)36-42(39,40)27-14-13-23(20-35-27)31(32,33)34/h5-14,18,20,25,36-37H,3-4,15-17,19H2,1-2H3/t25-,30-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease by fluorescent peptide substrate based assay |
Antimicrob Agents Chemother 51: 3147-54 (2007)
Article DOI: 10.1128/aac.00401-07
BindingDB Entry DOI: 10.7270/Q23R0WPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Antimicrob Agents Chemother 51: 3147-54 (2007)
Article DOI: 10.1128/aac.00401-07
BindingDB Entry DOI: 10.7270/Q23R0WPV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556532
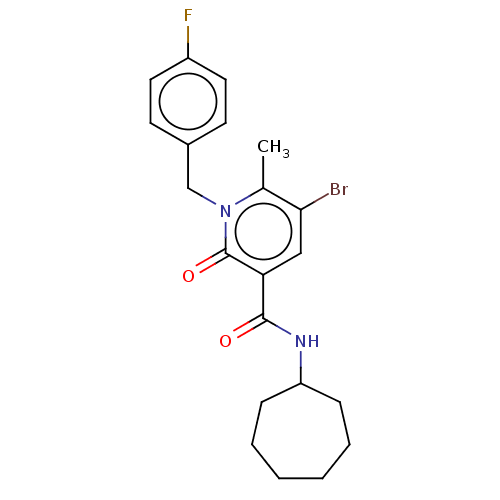
(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601131

(CHEMBL5193398)Show SMILES Cc1c(Br)cn(CCCc2cn(CCCCCn3c(C)c(Br)cc(C(=O)NC4CCCCCC4)c3=O)nn2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601132

(CHEMBL5204176)Show SMILES Cc1nc(OCCCCCn2cc(CCCn3cc(Br)c(C)c(NC(=O)C4CCCCCC4)c3=O)nn2)c(cc1Br)C(=O)NC1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556532

(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50562160
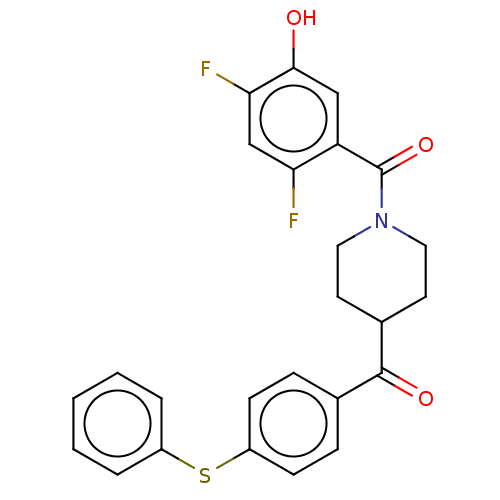
(CHEMBL4757403)Show SMILES Oc1cc(C(=O)N2CCC(CC2)C(=O)c2ccc(Sc3ccccc3)cc2)c(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... |
Bioorg Med Chem Lett 13: 1119-23 (2003)
Article DOI: 10.1016/j.ejmech.2020.112857
BindingDB Entry DOI: 10.7270/Q2D21X0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50570253

(CHEMBL4467500)Show SMILES Cc1c(Br)cn(Cc2ccc(F)cc2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50562158
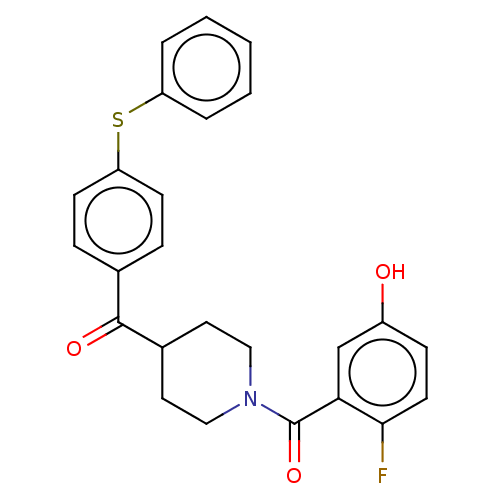
(CHEMBL4747585)Show SMILES Oc1ccc(F)c(c1)C(=O)N1CCC(CC1)C(=O)c1ccc(Sc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... |
Bioorg Med Chem Lett 13: 1119-23 (2003)
Article DOI: 10.1016/j.ejmech.2020.112857
BindingDB Entry DOI: 10.7270/Q2D21X0K |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50562161
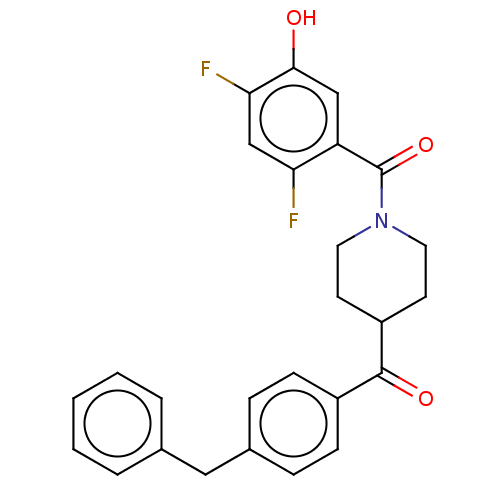
(CHEMBL4755606)Show SMILES Oc1cc(C(=O)N2CCC(CC2)C(=O)c2ccc(Cc3ccccc3)cc2)c(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... |
Bioorg Med Chem Lett 13: 1119-23 (2003)
Article DOI: 10.1016/j.ejmech.2020.112857
BindingDB Entry DOI: 10.7270/Q2D21X0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50562159

(CHEMBL4798508)Show SMILES Oc1ccc(F)c(c1)C(=O)N1CCC(CC1)C(=O)c1ccc(Cc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... |
Bioorg Med Chem Lett 13: 1119-23 (2003)
Article DOI: 10.1016/j.ejmech.2020.112857
BindingDB Entry DOI: 10.7270/Q2D21X0K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50601132

(CHEMBL5204176)Show SMILES Cc1nc(OCCCCCn2cc(CCCn3cc(Br)c(C)c(NC(=O)C4CCCCCC4)c3=O)nn2)c(cc1Br)C(=O)NC1CCCCCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50570253

(CHEMBL4467500)Show SMILES Cc1c(Br)cn(Cc2ccc(F)cc2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50601131

(CHEMBL5193398)Show SMILES Cc1c(Br)cn(CCCc2cn(CCCCCn3c(C)c(Br)cc(C(=O)NC4CCCCCC4)c3=O)nn2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50418490
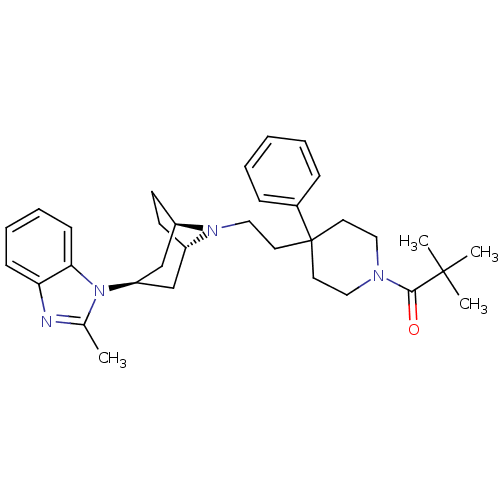
(CHEMBL1784385)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)C(C)(C)C)c1ccccc1 |r,THB:18:17:11.10.16:13.14| Show InChI InChI=1S/C33H44N4O/c1-24-34-29-12-8-9-13-30(29)37(24)28-22-26-14-15-27(23-28)36(26)21-18-33(25-10-6-5-7-11-25)16-19-35(20-17-33)31(38)32(2,3)4/h5-13,26-28H,14-23H2,1-4H3/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting |
J Med Chem 54: 3756-67 (2011)
Article DOI: 10.1021/jm200279v
BindingDB Entry DOI: 10.7270/Q26111K7 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331659
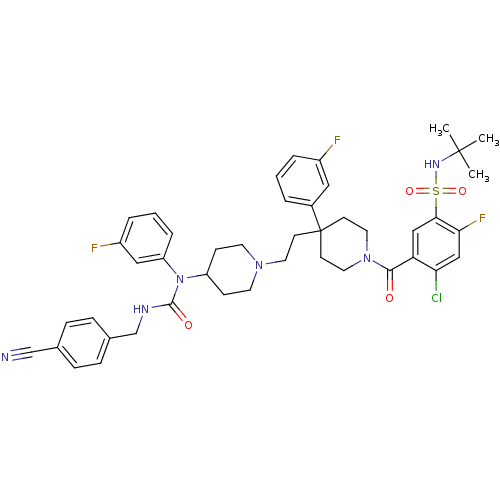
(CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(C(=O)NCc3ccc(cc3)C#N)c3cccc(F)c3)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C44H48ClF3N6O4S/c1-43(2,3)51-59(57,58)40-26-37(38(45)27-39(40)48)41(55)53-22-17-44(18-23-53,32-6-4-7-33(46)24-32)16-21-52-19-14-35(15-20-52)54(36-9-5-8-34(47)25-36)42(56)50-29-31-12-10-30(28-49)11-13-31/h4-13,24-27,35,51H,14-23,29H2,1-3H3,(H,50,56) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against wild-type reverse transcriptase of HIV-1 |
J Med Chem 47: 1175-82 (2004)
Article DOI: 10.1021/jm030255y
BindingDB Entry DOI: 10.7270/Q2TX3G3N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257737
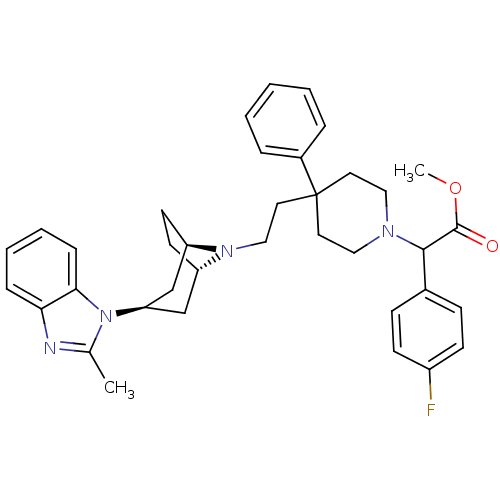
(CHEMBL444497 | methyl 2-(4-fluorophenyl)-2-(4-(2-(...)Show SMILES COC(=O)C(N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1)c1ccc(F)cc1 |r,THB:19:17:11:13.14| Show InChI InChI=1S/C37H43FN4O2/c1-26-39-33-10-6-7-11-34(33)42(26)32-24-30-16-17-31(25-32)41(30)23-20-37(28-8-4-3-5-9-28)18-21-40(22-19-37)35(36(43)44-2)27-12-14-29(38)15-13-27/h3-15,30-32,35H,16-25H2,1-2H3/t30-,31+,32+,35? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50412735
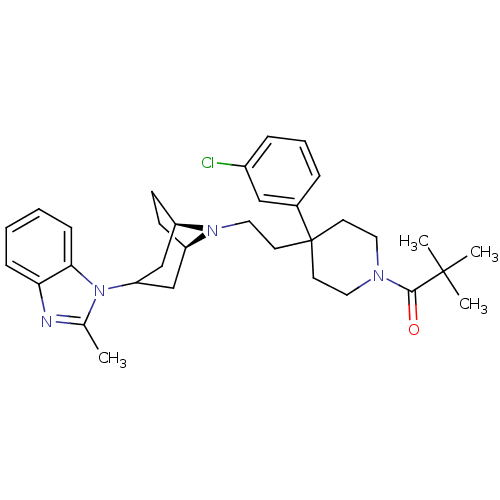
(CHEMBL454251)Show SMILES Cc1nc2ccccc2n1C1C[C@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)C(C)(C)C)c1cccc(Cl)c1 |r,TLB:9:10:17:13.14,THB:18:17:10.11.16:13.14| Show InChI InChI=1S/C33H43ClN4O/c1-23-35-29-10-5-6-11-30(29)38(23)28-21-26-12-13-27(22-28)37(26)19-16-33(24-8-7-9-25(34)20-24)14-17-36(18-15-33)31(39)32(2,3)4/h5-11,20,26-28H,12-19,21-22H2,1-4H3/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from human CCR5 expressed in CHO cells |
J Med Chem 51: 6538-46 (2008)
Article DOI: 10.1021/jm800598a
BindingDB Entry DOI: 10.7270/Q2WS8VG8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331659

(CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(C(=O)NCc3ccc(cc3)C#N)c3cccc(F)c3)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C44H48ClF3N6O4S/c1-43(2,3)51-59(57,58)40-26-37(38(45)27-39(40)48)41(55)53-22-17-44(18-23-53,32-6-4-7-33(46)24-32)16-21-52-19-14-35(15-20-52)54(36-9-5-8-34(47)25-36)42(56)50-29-31-12-10-30(28-49)11-13-31/h4-13,24-27,35,51H,14-23,29H2,1-3H3,(H,50,56) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257690
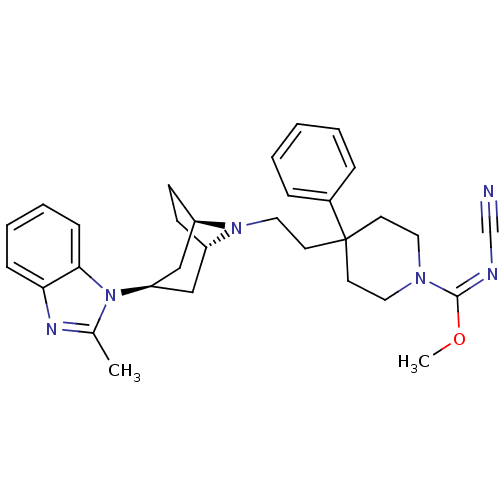
((E)-methyl N-cyano-4-(2-(3-(2-methyl-1H-benzo[d]im...)Show SMILES CO\C(=N\C#N)N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1 |r,THB:20:18:12:14.15| Show InChI InChI=1S/C31H38N6O/c1-23-34-28-10-6-7-11-29(28)37(23)27-20-25-12-13-26(21-27)36(25)19-16-31(24-8-4-3-5-9-24)14-17-35(18-15-31)30(38-2)33-22-32/h3-11,25-27H,12-21H2,1-2H3/b33-30+/t25-,26+,27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331658
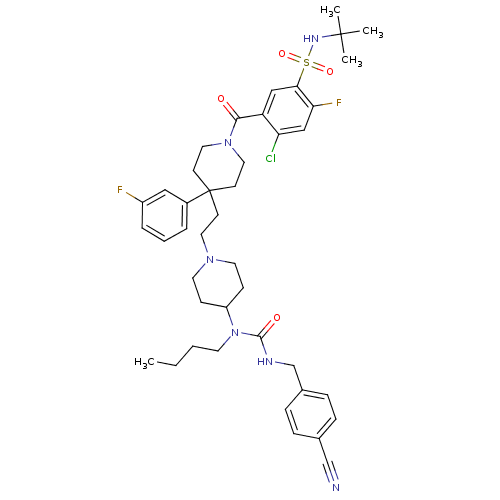
(CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...)Show SMILES CCCCN(C1CCN(CCC2(CCN(CC2)C(=O)c2cc(c(F)cc2Cl)S(=O)(=O)NC(C)(C)C)c2cccc(F)c2)CC1)C(=O)NCc1ccc(cc1)C#N Show InChI InChI=1S/C42H53ClF2N6O4S/c1-5-6-19-51(40(53)47-29-31-12-10-30(28-46)11-13-31)34-14-20-49(21-15-34)22-16-42(32-8-7-9-33(44)25-32)17-23-50(24-18-42)39(52)35-26-38(37(45)27-36(35)43)56(54,55)48-41(2,3)4/h7-13,25-27,34,48H,5-6,14-24,29H2,1-4H3,(H,47,53) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50483553

(CHEBI:76007 | Dolutegravir Sodium | GSK1349572 | G...)Show SMILES [Na;v0+].[H][C@]12[#6]-n3cc(-[#6](=O)-[#7]-[#6]-c4ccc(F)cc4F)c(=O)c(-[#8-])c3-[#6](=O)-[#7]1-[#6@H](-[#6])-[#6]-[#6]-[#8]2 |r| Show InChI InChI=1S/C20H19F2N3O5.Na/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22;/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28);/q;+1/p-1/t10-,15+;/m1./s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... |
Antimicrob Agents Chemother 55: 813-21 (2011)
Article DOI: 10.1128/AAC.01209-10
BindingDB Entry DOI: 10.7270/Q2RX9FXN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257691
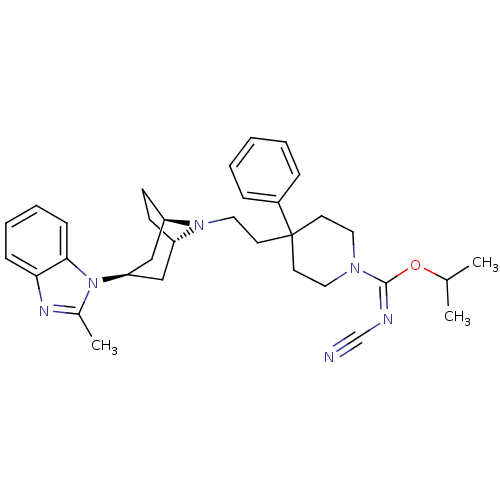
((E)-isopropyl N-cyano-4-(2-(3-(2-methyl-1H-benzo[d...)Show SMILES CC(C)O\C(=N\C#N)N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1 |r,THB:22:20:14:16.17| Show InChI InChI=1S/C33H42N6O/c1-24(2)40-32(35-23-34)37-18-15-33(16-19-37,26-9-5-4-6-10-26)17-20-38-27-13-14-28(38)22-29(21-27)39-25(3)36-30-11-7-8-12-31(30)39/h4-12,24,27-29H,13-22H2,1-3H3/b35-32+/t27-,28+,29+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257734

(2-(2,3-dimethylphenyl)-2-(4-(2-(3-(2-methyl-1H-ben...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(C(O)=O)c1cccc(C)c1C)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C38H46N4O2/c1-26-10-9-13-33(27(26)2)36(37(43)44)40-21-18-38(19-22-40,29-11-5-4-6-12-29)20-23-41-30-16-17-31(41)25-32(24-30)42-28(3)39-34-14-7-8-15-35(34)42/h4-15,30-32,36H,16-25H2,1-3H3,(H,43,44)/t30-,31+,32+,36? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1803
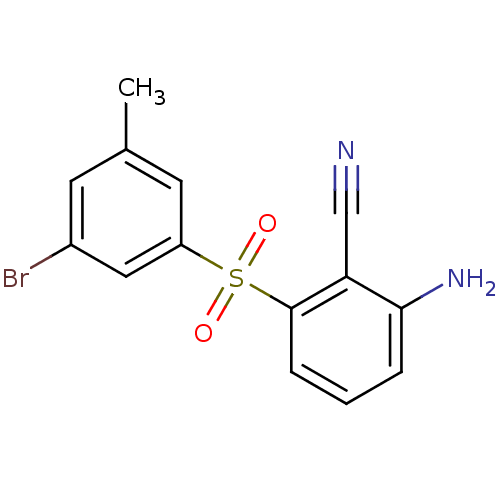
(2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...)Show InChI InChI=1S/C14H11BrN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257641
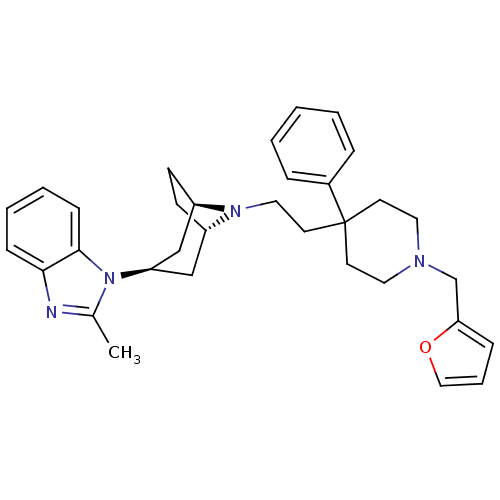
(1-(8-(2-(1-(furan-2-ylmethyl)-4-phenylpiperidin-4-...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(Cc2ccco2)CC1)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C33H40N4O/c1-25-34-31-11-5-6-12-32(31)37(25)29-22-27-13-14-28(23-29)36(27)20-17-33(26-8-3-2-4-9-26)15-18-35(19-16-33)24-30-10-7-21-38-30/h2-12,21,27-29H,13-20,22-24H2,1H3/t27-,28+,29+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM25351

(N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...)Show SMILES Cc1nnc(o1)C(=O)NC(C)(C)c1nc(C(=O)NCc2ccc(F)cc2)c(O)c(=O)n1C Show InChI InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human immunodeficiency virus 1 integrase by strand transfer scintillation proximity assay |
Antimicrob Agents Chemother 52: 901-8 (2008)
Article DOI: 10.1128/aac.01218-07
BindingDB Entry DOI: 10.7270/Q2JQ13VN |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50480673

(Isentress | Isentress hd | MK-0518 | MK-0518 POTAS...)Show SMILES [K;v0+].[#6]-c1nnc(o1)-[#6](=O)-[#7]C([#6])([#6])c1nc(-[#6](=O)-[#7]-[#6]-c2ccc(F)cc2)c(-[#8-])c(=O)n1-[#6] Show InChI InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30)/p-1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of strand transfer activity of Human immunodeficiency virus 1 Integrase using [3H]labeled target DNA as substrate after 45 mins by scintil... |
Antimicrob Agents Chemother 55: 813-21 (2011)
Article DOI: 10.1128/AAC.01209-10
BindingDB Entry DOI: 10.7270/Q2RX9FXN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331651

(CHEMBL1288917 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)Cc3ccc(cc3)C(F)(F)F)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C41H48ClF5N4O4S/c1-5-18-51(37(52)24-28-9-11-29(12-10-28)41(45,46)47)32-13-19-49(20-14-32)21-15-40(30-7-6-8-31(43)25-30)16-22-50(23-17-40)38(53)33-26-36(35(44)27-34(33)42)56(54,55)48-39(2,3)4/h5-12,25-27,32,48H,1,13-24H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331646
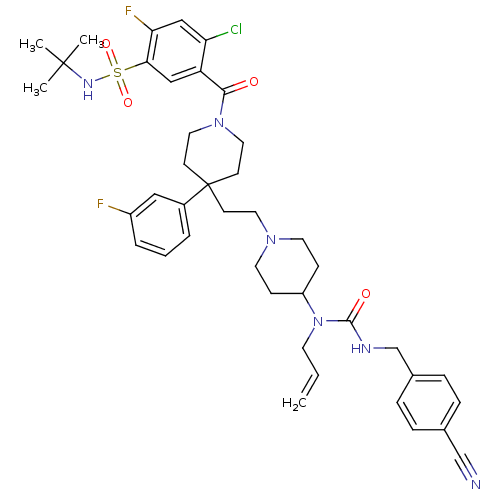
(5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)NCc3ccc(cc3)C#N)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C41H49ClF2N6O4S/c1-5-18-50(39(52)46-28-30-11-9-29(27-45)10-12-30)33-13-19-48(20-14-33)21-15-41(31-7-6-8-32(43)24-31)16-22-49(23-17-41)38(51)34-25-37(36(44)26-35(34)42)55(53,54)47-40(2,3)4/h5-12,24-26,33,47H,1,13-23,28H2,2-4H3,(H,46,52) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331646

(5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)NCc3ccc(cc3)C#N)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C41H49ClF2N6O4S/c1-5-18-50(39(52)46-28-30-11-9-29(27-45)10-12-30)33-13-19-48(20-14-33)21-15-41(31-7-6-8-32(43)24-31)16-22-49(23-17-41)38(51)34-25-37(36(44)26-35(34)42)55(53,54)47-40(2,3)4/h5-12,24-26,33,47H,1,13-23,28H2,2-4H3,(H,46,52) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257642
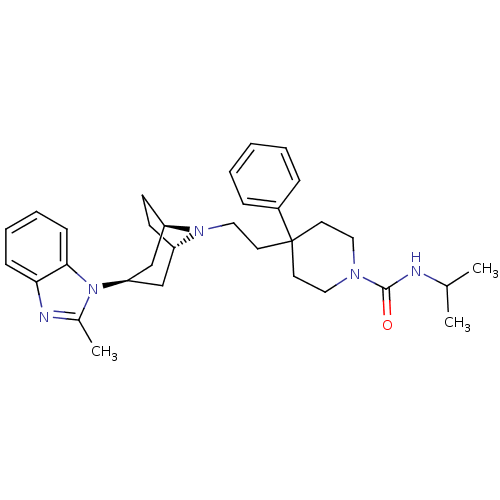
(CHEMBL524050 | N-isopropyl-4-(2-(3-(2-methyl-1H-be...)Show SMILES CC(C)NC(=O)N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1 |r,THB:20:18:12:14.15| Show InChI InChI=1S/C32H43N5O/c1-23(2)33-31(38)35-18-15-32(16-19-35,25-9-5-4-6-10-25)17-20-36-26-13-14-27(36)22-28(21-26)37-24(3)34-29-11-7-8-12-30(29)37/h4-12,23,26-28H,13-22H2,1-3H3,(H,33,38)/t26-,27+,28+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257731
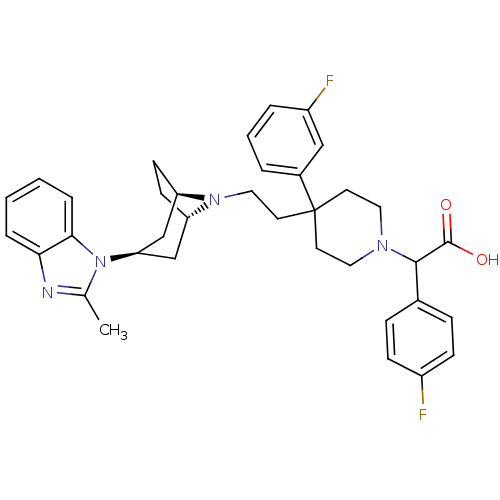
(2-(4-fluorophenyl)-2-(4-(3-fluorophenyl)-4-(2-(3-(...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(C(O)=O)c1ccc(F)cc1)c1cccc(F)c1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C36H40F2N4O2/c1-24-39-32-7-2-3-8-33(32)42(24)31-22-29-13-14-30(23-31)41(29)20-17-36(26-5-4-6-28(38)21-26)15-18-40(19-16-36)34(35(43)44)25-9-11-27(37)12-10-25/h2-12,21,29-31,34H,13-20,22-23H2,1H3,(H,43,44)/t29-,30+,31+,34? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331639

(4-cyanobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)OCc3ccc(cc3)C#N)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C41H48ClF2N5O5S/c1-5-18-49(39(51)54-28-30-11-9-29(27-45)10-12-30)33-13-19-47(20-14-33)21-15-41(31-7-6-8-32(43)24-31)16-22-48(23-17-41)38(50)34-25-37(36(44)26-35(34)42)55(52,53)46-40(2,3)4/h5-12,24-26,33,46H,1,13-23,28H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50331638

(4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...)Show SMILES CC(C)(C)NS(=O)(=O)c1cc(C(=O)N2CCC(CCN3CCC(CC3)N(CC=C)C(=O)OCc3ccc(cc3)S(C)(=O)=O)(CC2)c2cccc(F)c2)c(Cl)cc1F Show InChI InChI=1S/C41H51ClF2N4O7S2/c1-6-19-48(39(50)55-28-29-10-12-33(13-11-29)56(5,51)52)32-14-20-46(21-15-32)22-16-41(30-8-7-9-31(43)25-30)17-23-47(24-18-41)38(49)34-26-37(36(44)27-35(34)42)57(53,54)45-40(2,3)4/h6-13,25-27,32,45H,1,14-24,28H2,2-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection |
Bioorg Med Chem Lett 20: 7397-400 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.033
BindingDB Entry DOI: 10.7270/Q27081P6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257736

(CHEMBL506273 | methyl 2-(4-(2-(3-(2-methyl-1H-benz...)Show SMILES COC(=O)C(N1CCC(CCN2[C@H]3CC[C@@H]2C[C@@H](C3)n2c(C)nc3ccccc23)(CC1)c1ccccc1)c1ccccc1 |r,THB:19:17:11:13.14| Show InChI InChI=1S/C37H44N4O2/c1-27-38-33-15-9-10-16-34(33)41(27)32-25-30-17-18-31(26-32)40(30)24-21-37(29-13-7-4-8-14-29)19-22-39(23-20-37)35(36(42)43-2)28-11-5-3-6-12-28/h3-16,30-32,35H,17-26H2,1-2H3/t30-,31+,32+,35? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257733
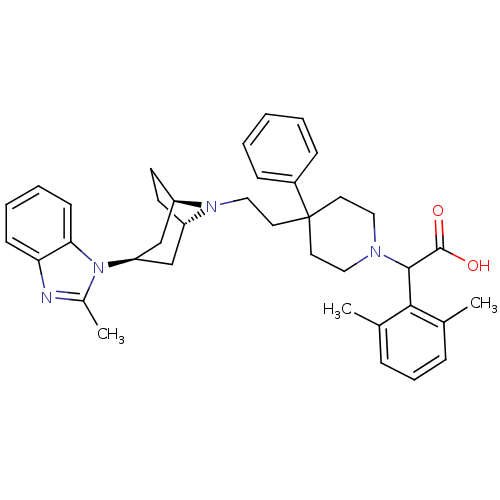
(2-(2,6-dimethylphenyl)-2-(4-(2-(3-(2-methyl-1H-ben...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(C(O)=O)c1c(C)cccc1C)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C38H46N4O2/c1-26-10-9-11-27(2)35(26)36(37(43)44)40-21-18-38(19-22-40,29-12-5-4-6-13-29)20-23-41-30-16-17-31(41)25-32(24-30)42-28(3)39-33-14-7-8-15-34(33)42/h4-15,30-32,36H,16-25H2,1-3H3,(H,43,44)/t30-,31+,32+,36? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1804
(2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...)Show InChI InChI=1S/C14H11ClN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257728
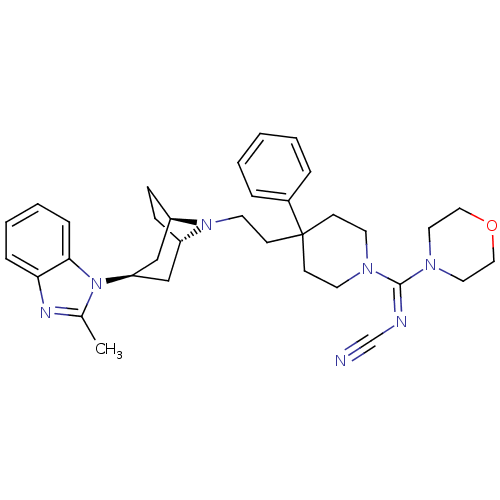
((E)-N-((4-(2-(3-(2-methyl-1H-benzo[d]imidazol-1-yl...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=N/C#N)\N1CCOCC1)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C34H43N7O/c1-26-37-31-9-5-6-10-32(31)41(26)30-23-28-11-12-29(24-30)40(28)18-15-34(27-7-3-2-4-8-27)13-16-38(17-14-34)33(36-25-35)39-19-21-42-22-20-39/h2-10,28-30H,11-24H2,1H3/b36-33+/t28-,29+,30+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50412762
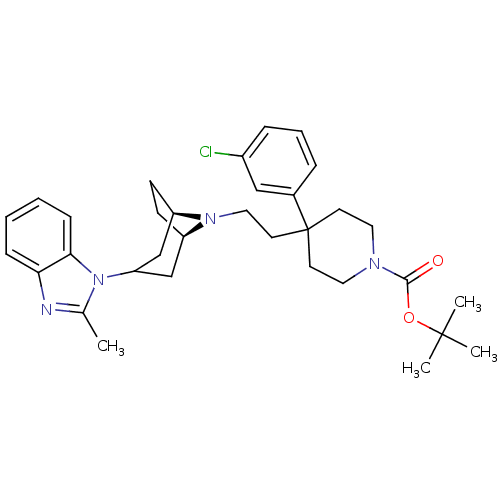
(CHEMBL444938)Show SMILES Cc1nc2ccccc2n1C1C[C@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)OC(C)(C)C)c1cccc(Cl)c1 |r,TLB:9:10:17:13.14,THB:18:17:10.11.16:13.14| Show InChI InChI=1S/C33H43ClN4O2/c1-23-35-29-10-5-6-11-30(29)38(23)28-21-26-12-13-27(22-28)37(26)19-16-33(24-8-7-9-25(34)20-24)14-17-36(18-15-33)31(39)40-32(2,3)4/h5-11,20,26-28H,12-19,21-22H2,1-4H3/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from human CCR5 expressed in CHO cells |
J Med Chem 51: 6538-46 (2008)
Article DOI: 10.1021/jm800598a
BindingDB Entry DOI: 10.7270/Q2WS8VG8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50418505
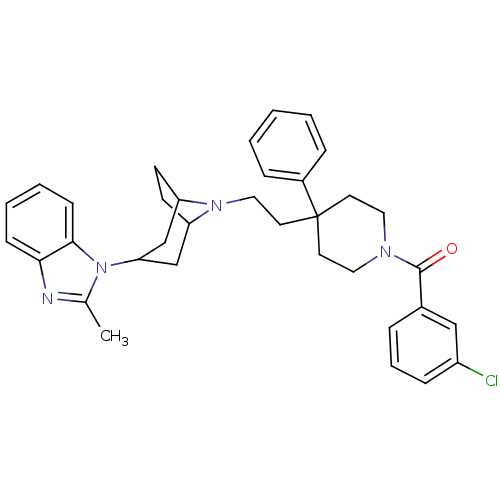
(CHEMBL1784481)Show SMILES Cc1nc2ccccc2n1C1CC2CCC(C1)N2CCC1(CCN(CC1)C(=O)c1cccc(Cl)c1)c1ccccc1 Show InChI InChI=1S/C35H39ClN4O/c1-25-37-32-12-5-6-13-33(32)40(25)31-23-29-14-15-30(24-31)39(29)21-18-35(27-9-3-2-4-10-27)16-19-38(20-17-35)34(41)26-8-7-11-28(36)22-26/h2-13,22,29-31H,14-21,23-24H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting |
J Med Chem 54: 3756-67 (2011)
Article DOI: 10.1021/jm200279v
BindingDB Entry DOI: 10.7270/Q26111K7 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50412739
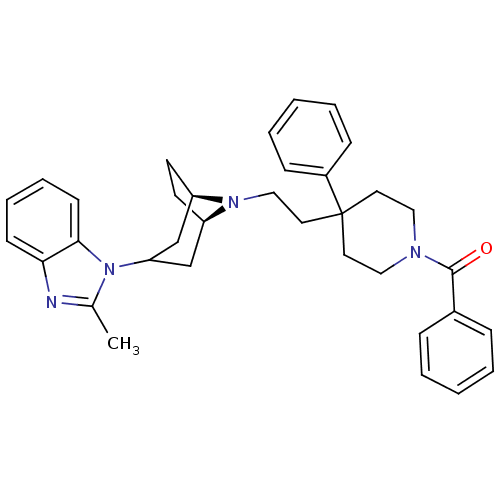
(CHEMBL503033)Show SMILES Cc1nc2ccccc2n1C1C[C@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)c1ccccc1)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C35H40N4O/c1-26-36-32-14-8-9-15-33(32)39(26)31-24-29-16-17-30(25-31)38(29)23-20-35(28-12-6-3-7-13-28)18-21-37(22-19-35)34(40)27-10-4-2-5-11-27/h2-15,29-31H,16-25H2,1H3/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from human CCR5 expressed in CHO cells |
J Med Chem 51: 6538-46 (2008)
Article DOI: 10.1021/jm800598a
BindingDB Entry DOI: 10.7270/Q2WS8VG8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50412738
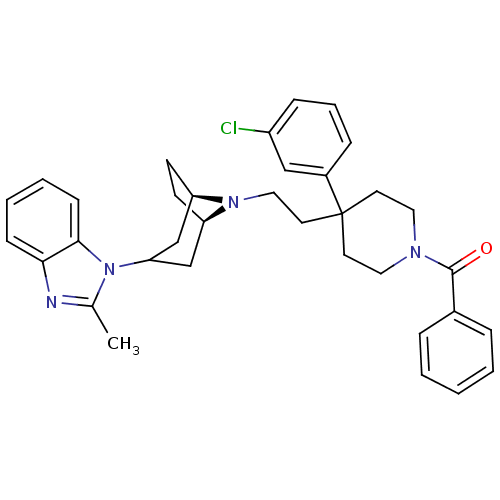
(CHEMBL445772)Show SMILES Cc1nc2ccccc2n1C1C[C@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)c1ccccc1)c1cccc(Cl)c1 |r,TLB:9:10:17:13.14,THB:18:17:10.11.16:13.14| Show InChI InChI=1S/C35H39ClN4O/c1-25-37-32-12-5-6-13-33(32)40(25)31-23-29-14-15-30(24-31)39(29)21-18-35(27-10-7-11-28(36)22-27)16-19-38(20-17-35)34(41)26-8-3-2-4-9-26/h2-13,22,29-31H,14-21,23-24H2,1H3/t29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from human CCR5 expressed in CHO cells |
J Med Chem 51: 6538-46 (2008)
Article DOI: 10.1021/jm800598a
BindingDB Entry DOI: 10.7270/Q2WS8VG8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50257644
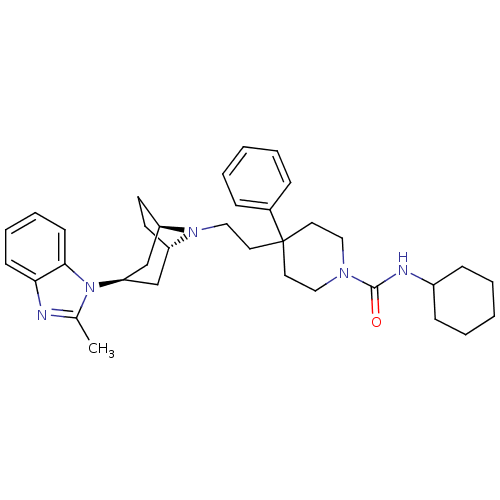
(CHEMBL471149 | N-cyclohexyl-4-(2-(3-(2-methyl-1H-b...)Show SMILES Cc1nc2ccccc2n1[C@@H]1C[C@@H]2CC[C@H](C1)N2CCC1(CCN(CC1)C(=O)NC1CCCCC1)c1ccccc1 |r,TLB:9:10:17:13.14| Show InChI InChI=1S/C35H47N5O/c1-26-36-32-14-8-9-15-33(32)40(26)31-24-29-16-17-30(25-31)39(29)23-20-35(27-10-4-2-5-11-27)18-21-38(22-19-35)34(41)37-28-12-6-3-7-13-28/h2,4-5,8-11,14-15,28-31H,3,6-7,12-13,16-25H2,1H3,(H,37,41)/t29-,30+,31+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1beta from CCR5 (unknown origin) expressed in CHO cell membrane |
Bioorg Med Chem Lett 19: 1610-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.014
BindingDB Entry DOI: 10.7270/Q2VQ32JR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data