

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418490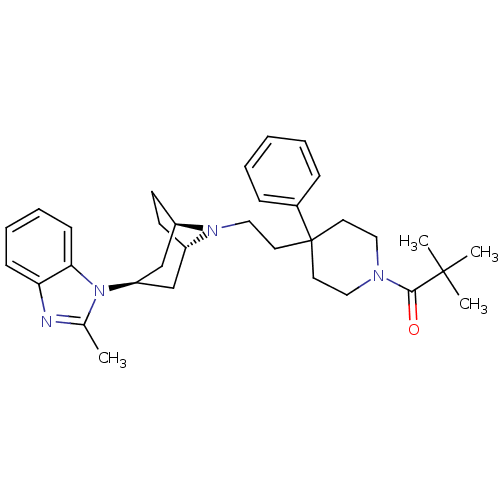 (CHEMBL1784385) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659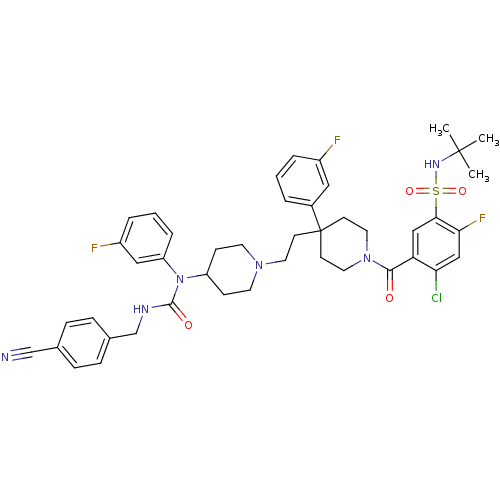 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331659 (CHEMBL1288663 | N-tert-butyl-4-chloro-5-(4-(2-(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8686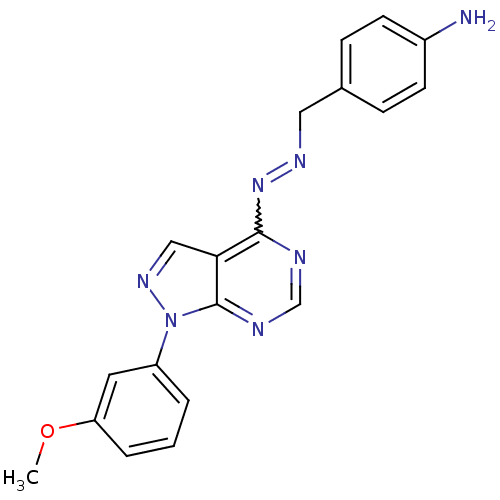 (4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8688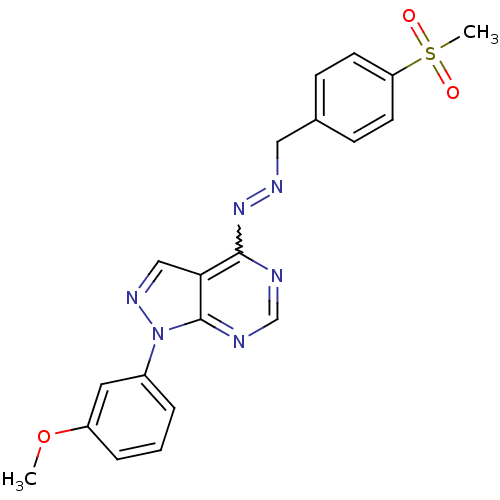 ((1E)-1-[(4-methanesulfonylphenyl)methylidene]-2-[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331658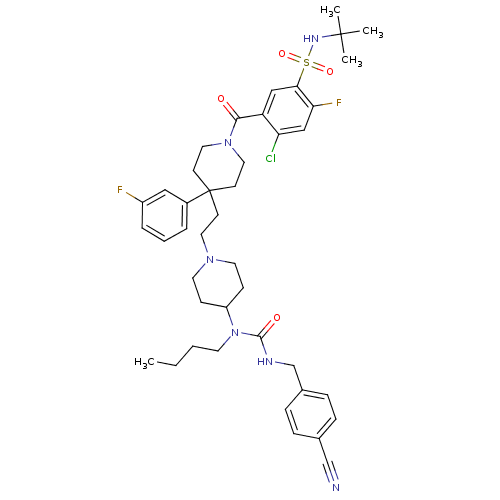 (CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8674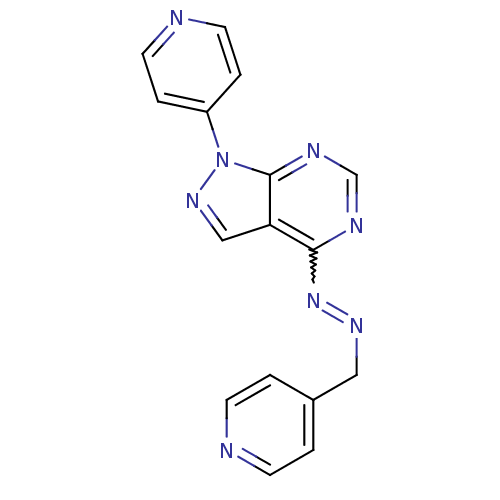 (4-{4-[(2E)-2-(pyridin-4-ylmethylidene)hydrazin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331651 (CHEMBL1288917 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646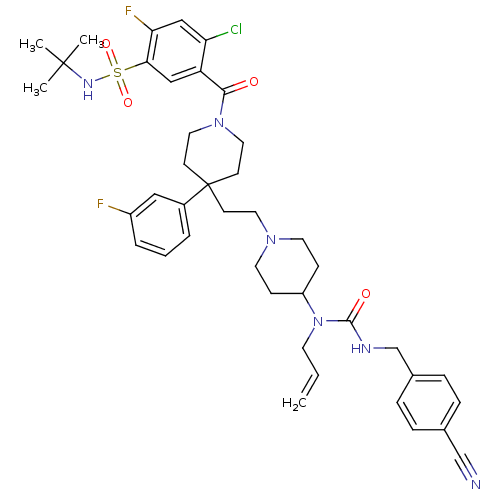 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331646 (5-(4-(2-(4-(1-allyl-3-(4-cyanobenzyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8695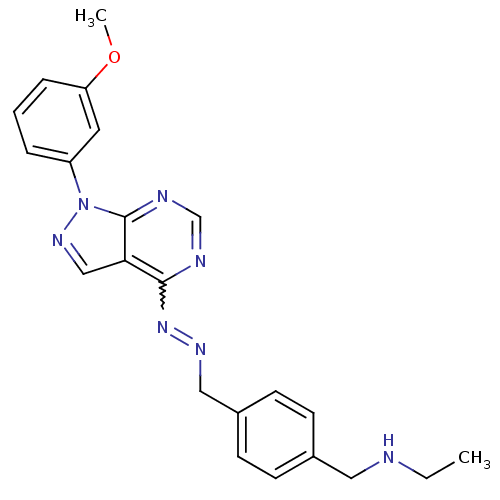 (ethyl({4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8687 ((1E)-1-[(3-methanesulfonylphenyl)methylidene]-2-[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331639 (4-cyanobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331638 (4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8698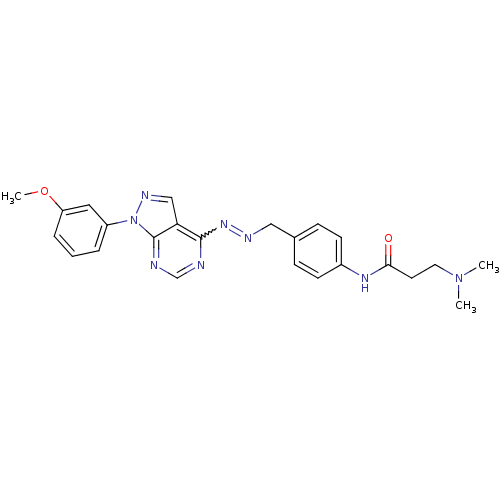 (3-(dimethylamino)-N-{4-[(1E)-{2-[1-(3-methoxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418505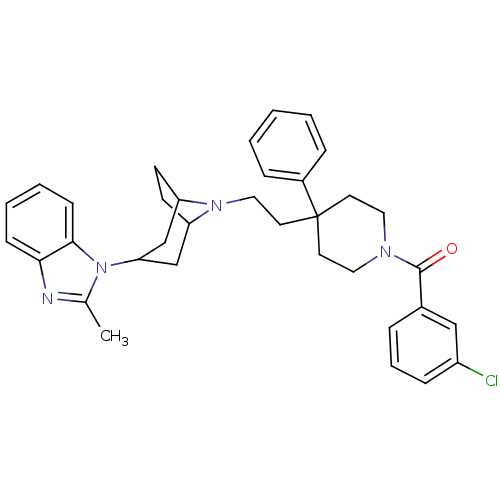 (CHEMBL1784481) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8694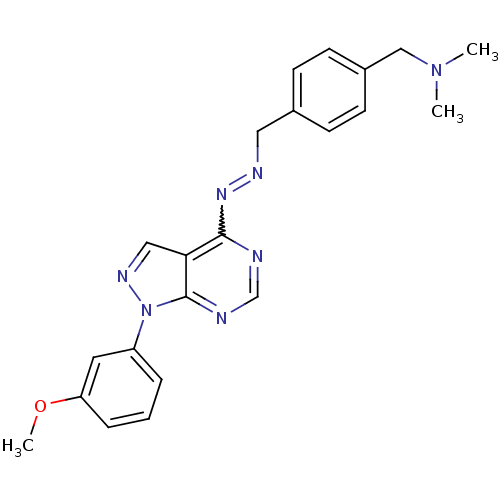 (({4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8692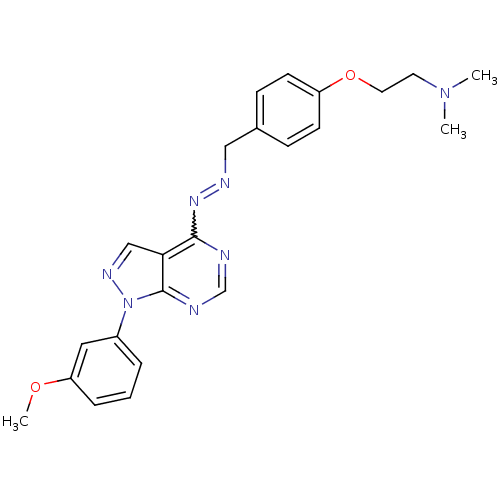 ((2-{4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331642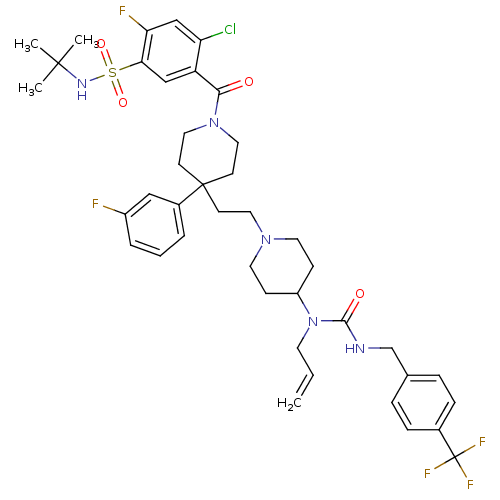 (5-(4-(2-(4-(1-allyl-3-(4-(trifluoromethyl)benzyl)u...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8704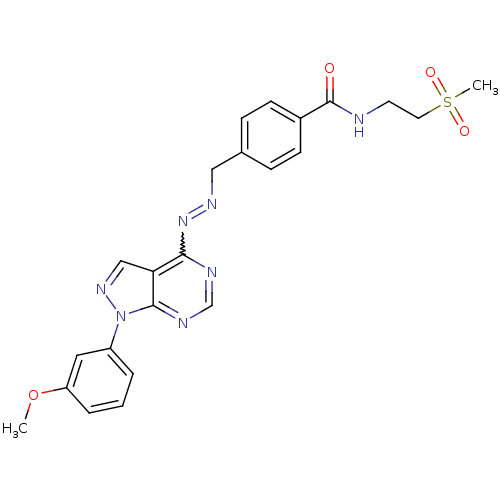 (N-(2-methanesulfonylethyl)-4-[(1E)-{2-[1-(3-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8682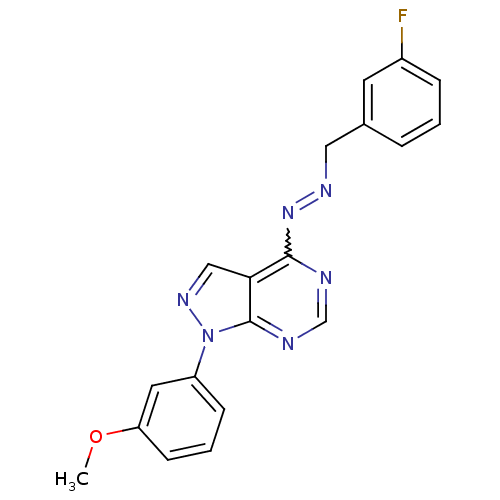 ((1E)-1-[(3-fluorophenyl)methylidene]-2-[1-(3-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8683 ((1E)-1-[(4-fluorophenyl)methylidene]-2-[1-(3-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331660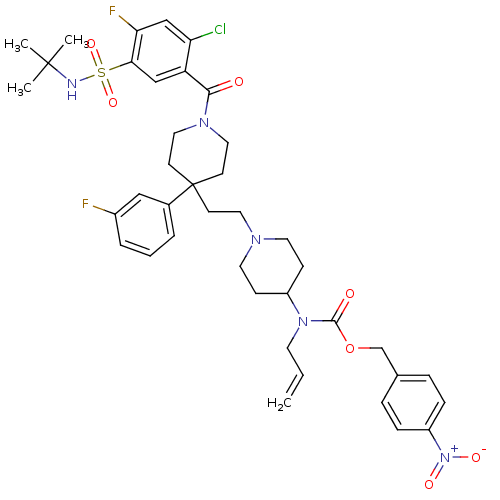 (4-nitrobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418547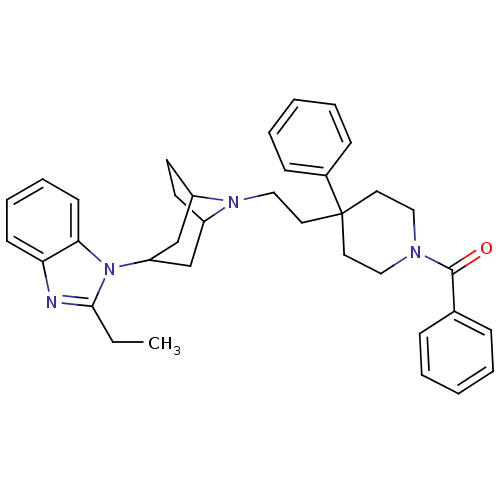 (CHEMBL1784404) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8708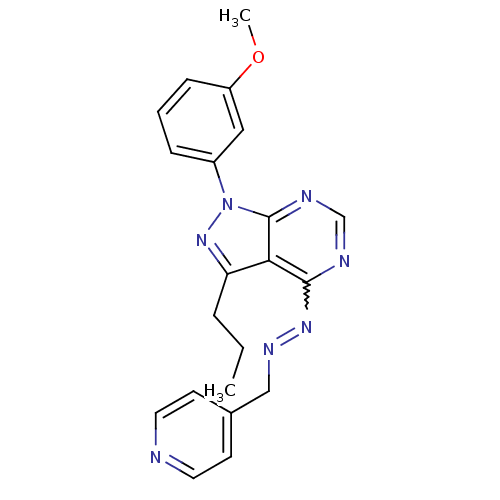 (4-[(1E)-{2-[1-(3-methoxyphenyl)-3-propyl-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8706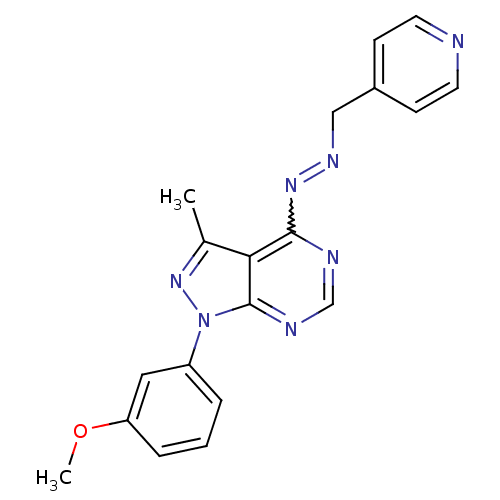 (4-[(1E)-{2-[1-(3-methoxyphenyl)-3-methyl-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8705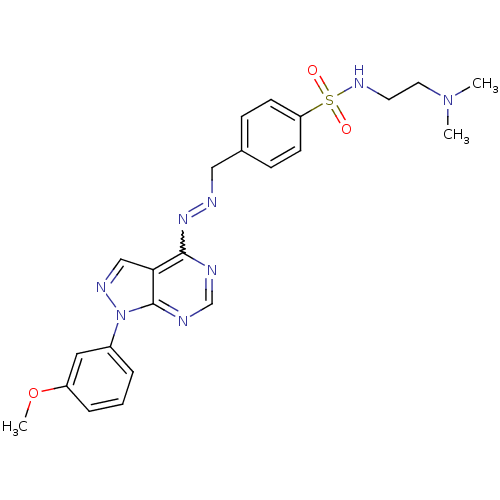 (N-[2-(dimethylamino)ethyl]-4-[(1E)-{2-[1-(3-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8685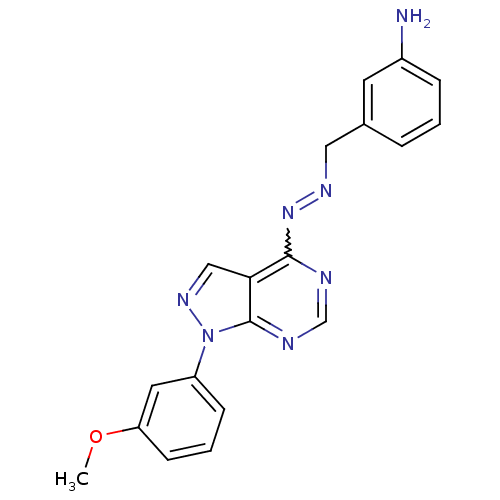 (3-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8677 ((2E)-1-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418499 (CHEMBL1784400) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331658 (CHEMBL1288924 | N-tert-butyl-5-(4-(2-(4-(1-butyl-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331647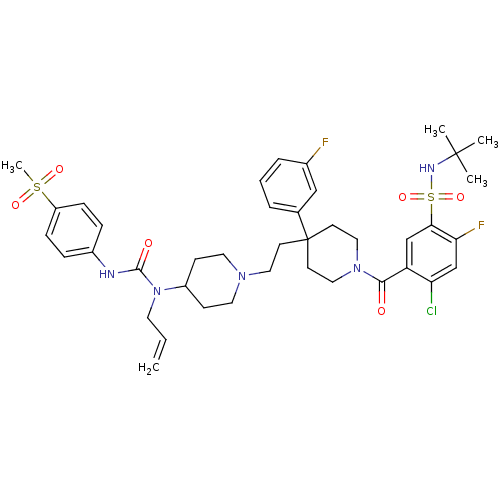 (5-(4-(2-(4-(1-allyl-3-(4-(methylsulfonyl)phenyl)ur...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8689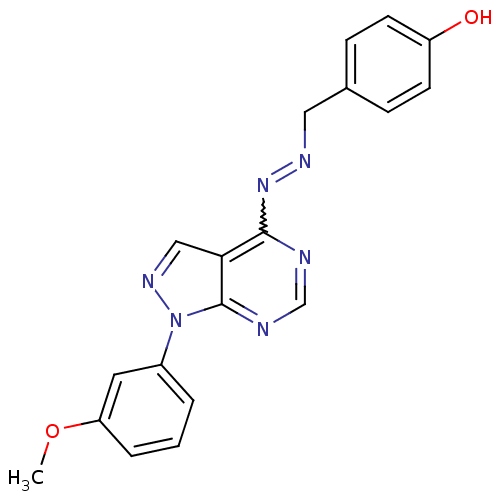 (4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8707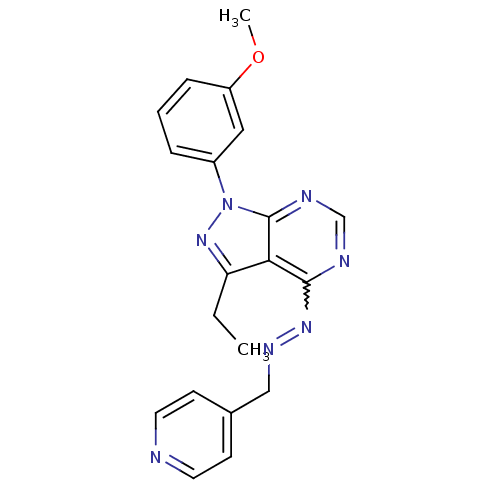 (4-[(1E)-{2-[3-ethyl-1-(3-methoxyphenyl)-1H-pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8696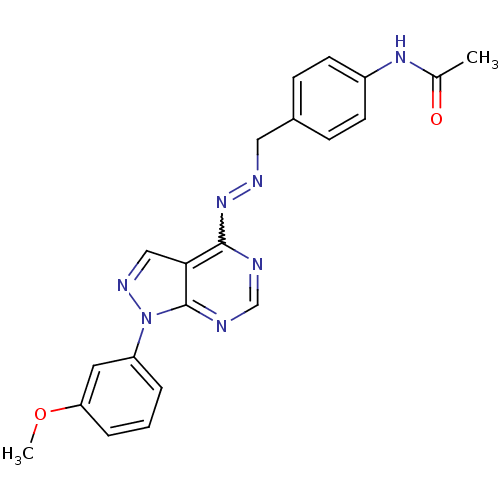 (N-{4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331661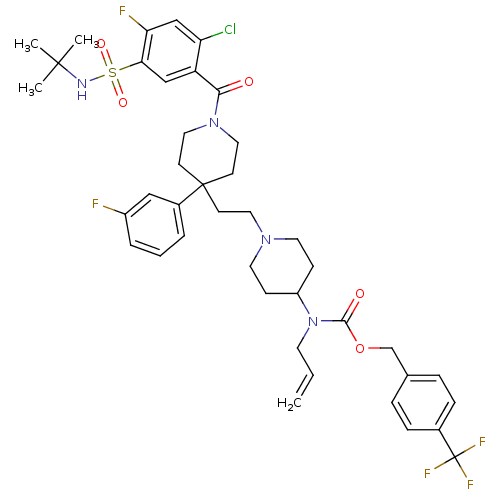 (4-(trifluoromethyl)benzyl allyl(1-(2-(1-(5-(N-tert...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418489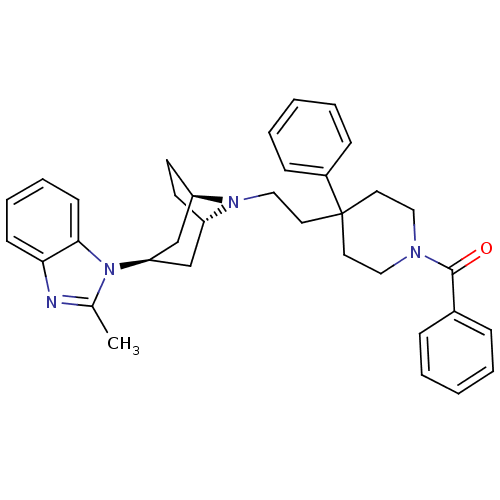 (CHEMBL1784384) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8684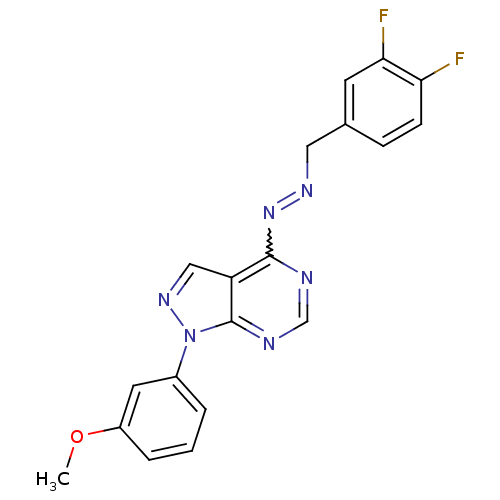 ((1E)-1-[(3,4-difluorophenyl)methylidene]-2-[1-(3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418541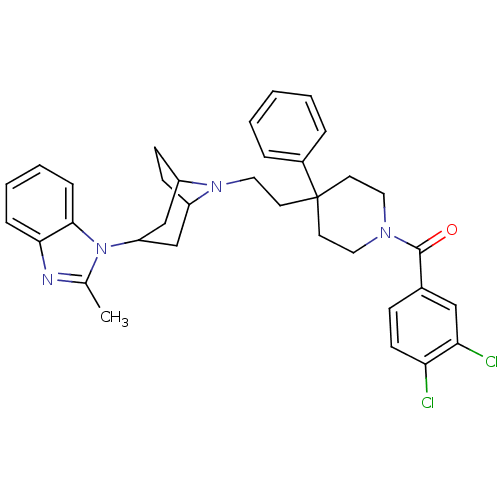 (CHEMBL1784479) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8701 (4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4-d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8703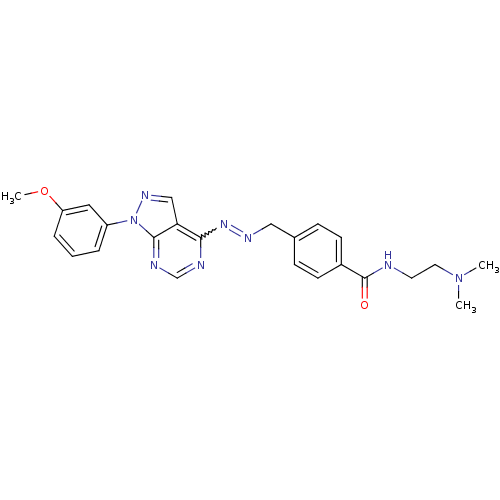 (N-[2-(dimethylamino)ethyl]-4-[(1E)-{2-[1-(3-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8697 (N-{4-[(1E)-{2-[1-(3-methoxyphenyl)-1H-pyrazolo[3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af... | Bioorg Med Chem Lett 14: 2121-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.036 BindingDB Entry DOI: 10.7270/Q2NV9GF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331638 (4-(methylsulfonyl)benzyl allyl(1-(2-(1-(5-(N-tert-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418548 (CHEMBL1784483) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331654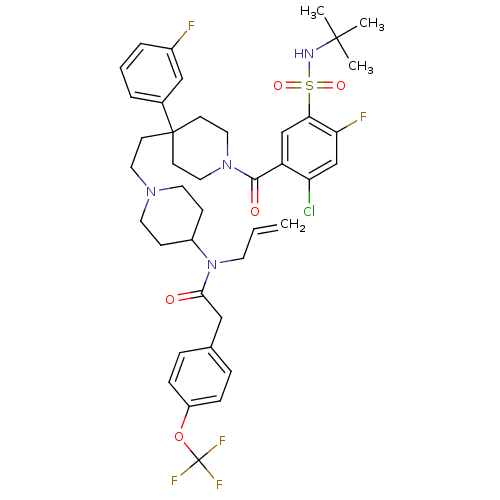 (CHEMBL1288920 | N-allyl-N-(1-(2-(1-(5-(N-tert-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50418500 (CHEMBL1784401) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of 125I-MIP-1beta from human CCR5 receptor after 4 hrs by scintillation counting | J Med Chem 54: 3756-67 (2011) Article DOI: 10.1021/jm200279v BindingDB Entry DOI: 10.7270/Q26111K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331645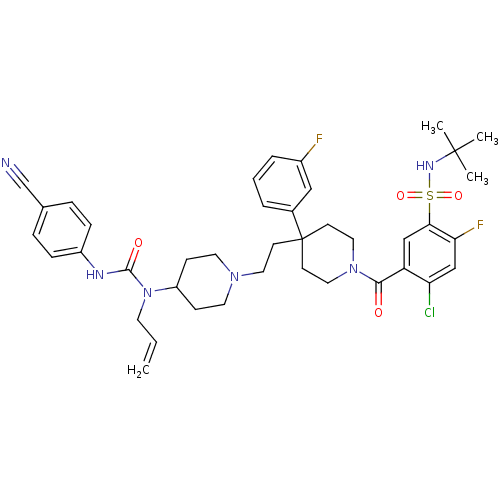 (5-(4-(2-(4-(1-allyl-3-(4-cyanophenyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331662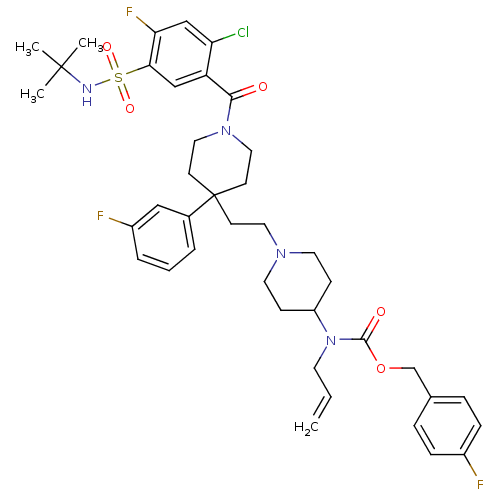 (4-fluorobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in HOS cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331660 (4-nitrobenzyl allyl(1-(2-(1-(5-(N-tert-butylsulfam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50331645 (5-(4-(2-(4-(1-allyl-3-(4-cyanophenyl)ureido)piperi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human peripheral blood lymphocytes cells assessed as inhibition of HIV-1 Ba-L infection | Bioorg Med Chem Lett 20: 7397-400 (2010) Article DOI: 10.1016/j.bmcl.2010.10.033 BindingDB Entry DOI: 10.7270/Q27081P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 168 total ) | Next | Last >> |